|
|
|
Indian Pediatr
2017;54:1012-1016 |
 |
Prevalence of
Nonalcoholic Fatty Liver Disease in Normal-weight and Overweight
Preadolescent Children in Haryana, India
|
|
Manoja Kumar Das, #Vidyut
Bhatia, #Anupam
Sibal, *Abha
Gupta, #Sarath
Gopalan,
‡Raman Sardana, $Reeti
Sahni, #Ankur Roy
and Narendra K Arora
From The INCLEN Trust International, F1/5, Okhla
Industrial Area, Phase I, New Delhi; and Departments of #Pediatric
Gastroenterology and Hepatology, *Biochemistry, ‡Microbiology
and $Radiodiagnosis, Indraprastha Apollo Hospitals, Sarita
Vihar, New Delhi; India.
Correspondence to : Dr Manoja Kumar Das, MD, Director
Projects, The INCLEN Trust International, F1/5, Okhla Industrial Area,
Phase I, New Delhi 110020, India.
Email: [email protected]
Received: December 10, 2016;
Initial review completed: February 20, 2017;
Accepted: September 29, 2017.
|
Objective: To document the prevalence of
non-alcoholic fatty liver disease (NAFLD) and metabolic parameters among
normal-weight and overweight schoolchildren.
Study design: Cross-sectional study.
Setting: Thirteen private schools in urban
Faridabad, Haryana.
Participants: 961 school children aged
5-10 years.
Methods: Ultrasound testing was done, and 215
with fatty liver on ultrasound underwent further clinical, biochemical
and virological testing.
Outcome measures: Prevalence of fatty liver on
ultrasound, and NAFLD and its association with biochemical abnormalities
and demographic risk factors.
Results: On ultrasound, 215 (22.4%) children had
fatty liver; 18.9% in normal-weight and 45.6% in overweight category.
Presence and severity of fatty liver disease increased with body mass
index (BMI) and age. Among the children with NAFLD, elevated SGOT and
SGPT was observed in 21.5% and 10.4% children, respectively. Liver
enzyme derangement was significantly higher in overweight children (27%
vs 19.4% in normal-weight) and severity of fatty liver (28% vs
20% in mild fatty liver cases). Eleven (8.1%) children with NAFLD had
metabolic syndrome. Higher BMI (OR 35.9), severe fatty liver disease (OR
1.7) and female sex (OR 1.9) had strong association with metabolic
syndrome.
Conclusions: 22.4% of normal-weight and
overweight children aged 5-10 years had fatty liver. A high proportion
(18.9%) of normal-weight children with fatty liver on ultrasound
indicates the silent burden in the population.
Key words: Fatty Liver Disease, Metabolic Syndrome, NAFLD.
|
|
N
on-alcoholic fatty liver disease (NAFLD) is
defined as the accumulation of fat in the hepatocytes (>5-10% by weight)
in the absence of any known liver pathology or excessive alcohol
consumption [1]. NAFLD is the commonest cause of chronic liver disease
in young people in the developed countries [2,3]. Paralleling
overweight/obesity epidemiology, NAFLD report is also rising. Prevalence
of fatty liver in the general adult population of India is in the range
of 5% to 28%, with higher prevalence among the overweight and diabetics
[4-6]. Prevalence of childhood NAFLD globally is in the range of 9 to
37% [7,8]. Further, the prevalence of NAFLD in normal-weight children
and overweight/obese children is reported to range about 3-10% and
8-80%, respectively [9]. Presence of NAFLD varies according to the
methodology and diagnostic criteria used [10]. Overweight/Obesity,
dyslipidemia, insulin resistance and metabolic syndrome, ethnicity,
gender and familial clustering are documented as risk factors for NAFLD
in adults and children [9,11,12].
The increasing burden of obesity and non-communicable
diseases across all ages warrants documentation of NAFLD burden. There
is limited information about NAFLD among Indian preadolescent children.
We undertook this study to document the prevalence of NAFLD among urban
normal-weight and overweight school children in a northern Indian state,
and its association with insulin resistance and other components of
metabolic syndrome.
Methods
This was a cross-sectional study that included urban
school children aged ³5
years and £10
years from Faridabad district, Haryana. We adopted intelligent two stage
sampling: (1) selection of schools and (2) selection of suitable
children from the schools. Thirteen private schools were identified
randomly from the list of schools with District Education Office. After
obtaining informed written consent from parents, all the children in
eligible age group were screened for weight, height and blood pressure.
Weight and height were measured using electronic weighing scale
(precision 100 grams) and Leicester Stadiometer (precision 0.1 cm), and
Body mass index (BMI) calculated. Blood pressure was measured using
electronic sphygmomanometer after resting for 10 minutes. Three measures
for child each were obtained and mean of the measures was used.
Date of birth was verified from the school register. Using the
International Obesity Task Force (IOTF) reference for BMI, the children
were categorized into underweight, normal-weight and overweight (BMI
corresponding to <18.5, ³18.5-<25
and ³25 at 18
years of age, respectively) [13,14]. Children falling in the underweight
category were excluded to avoid influence of fatty liver associated with
undernutrition.
Ultrasonography (USG) abdomen was done at
Indraprastha Apollo hospital by a radiologist using Sonosite (Fujifilm
Sonosite Inc.) machine with 3.5 MHz convex-type transducer among
randomly selected children from the above. All the ultrasound images
were saved and reviewed by senior radiologist, and repeated in 10% of
the children. Ultrasound findings were categorized into absent, mild,
moderate and severe fatty liver disease, as per Needleman criteria [15].
Parents of the children with fatty liver on
ultrasound were approached for consent for second part of study for
detailed clinical examination and anthropometry, blood biochemistry, and
virological evaluation. Waist circumference and hip circumference were
measured using non-stretchable tape (Seca 201). Waist circumference
above 90 th percentile for
the age and gender was considered as abnormal [16]. Children with
hypertension were identified using the BP reference given by Manu Raj,
et al. [17]. Fasting venous blood sample (6 mL) was drawn for all
enrolled children, serum was separated within 2 hours and analyzed
within 24 hours. Blood glucose, liver function tests and lipid profile
were measured. Serum ceruloplasmin was analyzed by the
immunoturbidimetry method. Hepatitis B antigen (HBsAg) and
Anti-hepatitis C (HCV) assays were done by ELISA using kits (VIDAS
Biomeriex, France and Orthodiagnostics, USA, respectively). Internal
and external quality control protocols for continuous quality assurance
was adopted in the laboratory. Alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) >40 IU/L were considered as raised.
Metabolic syndrome was defined by abnormality of any three of the
parameters: fasting glucose >100 mg/dL, triglyceride >110 mg/dL,
high-density lipoprotein (HDL) <38 mg/dL, hypertension (blood pressure
>95th percentile for systolic blood pressure/diastolic blood
pressure/mean arterial blood pressure) and obesity (>95th percentile
BMI) [18].
Assuming prevalence of NAFLD in normal-weight and
overweight children to be in the range of 12% and 55% (relative
admissible error ±20%) at 95% confidence level, the sample size needed
was 704 and 80, respectively. Considering the estimated prevalence of
overweight in the range of 12-15%, a sample size of 960 children for
ultrasound was planned.
The study protocol was approved by the Institutional
Ethics Committees of both Inclen Trust International and Indraprastha
Apollo Hospital. Data analysis was done by STATA using descriptive
statistics (means and standard deviations), Mann-Whitney Test, unpaired
t-test and chi-square test. A P value less than 0.05 was
considered significant.
Results
A total of 3560 children aged
³5 and <10 years
(2087 boys and 1473 girls) were screened. Out of the children screened,
2092 were normal-weight, 402 were overweight and 154 were obese. A total
of 961 children (528 boys) underwent ultrasound examination (Fig.
1), out of which 836 were in normal-weight category and 125 in
overweight category. There was comparable representation from age-bands
among the children who underwent ultrasound and those which did not.
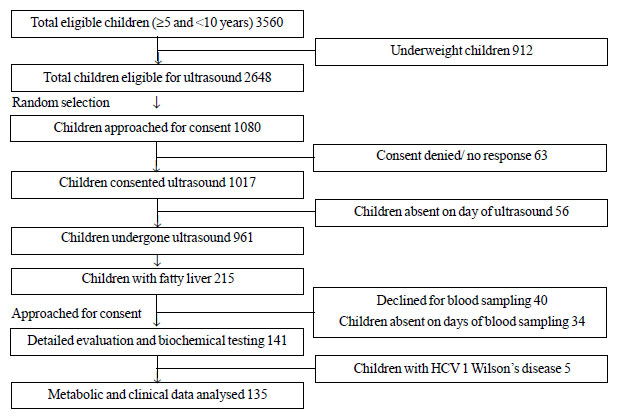 |
|
Fig. 1 Study participation flow chart.
|
Out of these 215 (22.4%; boys 25.6% and girls 18.5%)
had fatty liver on ultrasound. Out of the 215 children with fatty liver,
110 had mild degree and 25 had moderate degree and none had severe
degree. The prevalence of fatty liver increased with age in both boys
and girls, from 13.1% in 5-6 years to 31% in 9-10 years age-group (Fig.
2). Boys had a higher risk of fatty liver (OR 1.5; 95% CI 1.3-1.8).
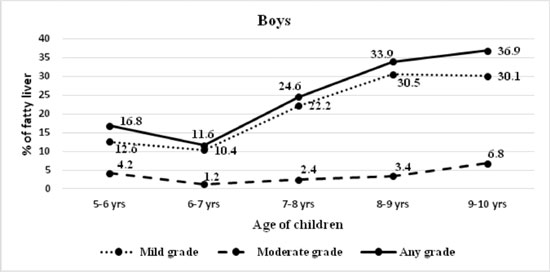
(a) |
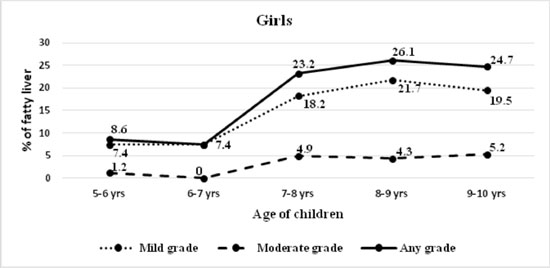
(b) |
|
Fig. 2 Severity of fatty liver
diagnosed on ultrasound in (a) boys; and (b) girls.
|
Proportion of fatty liver among overweight children
was 45.6%, twice than the normal-weight children (18.9%); which was
similar in both boys (48.5% and 22.3%, respectively) and girls (42.4%
and 14.7%, respectively). Higher BMI was observed to be a risk factor
for fatty liver in both sexes [boys, OR 3.28 (95% CI 2.96-3.64); girls,
OR 4.26 (95% CI 3.96-4.52); all children OR 3.59 (95% CI 2.9- 4.5)].
Only few children had any symptoms and/or signs like pain abdomen
(6.4%), jaundice (2.8%) or vomiting (2.1%). Family history of
hypertension, diabetes and liver disease were present in 12.8%, 6.4% and
3.5% of children, respectively and there was no relationship with
severity of fatty liver.
Detailed biochemical evaluation could be done in 141
children. AST and ALT levels were raised in 29 (21.5%) and 14 (10.4%)
children, respectively. AST level was elevated in 19.4% of normal-weight
children and 27% of the overweight children. SGPT level was elevated in
35% of overweight children and 1% of the normal-weight children.
TABLE I Metabolic Parameters in Children with Fatty Liver
|
Parameters |
Fatty liver, n(%) |
|
Mild
(n=109) |
Moderate
(n=26) |
Total
(n=135) |
|
Obese |
29 (26.6) |
8 (30.8) |
37 (27.4) |
|
Hypertension |
10 (9.2) |
3 (11.5) |
13 (9.6) |
|
Hyperglycemia |
13 (11.9) |
3 (11.5) |
16 (11.8) |
|
Hypertriglyceridemia |
26 (23.8) |
6 (23) |
32 (23.7) |
|
Low HDL |
39 (35.8) |
13 (50) |
52 (38.5) |
|
*Metabolic syndrome |
8 (7.3) |
3 (11.5) |
11 (8.1) |
|
*Metabolic syndrome was defined as presence of any three of
the five parameters. |
Low HDL (38.5%) and hypertriglyceridemia (23.7%) were
the commonest metabolic derangements observed (Table I).
The 11 children with metabolic syndrome had hypertriglyceridemia (100%),
obesity (91%), and low HDL (82%) as prominent features. When compared
across the BMI categories; low HDL level was comparable among
normal-weight (37.8%) and overweight children (38.1%), but increased
among obese children (43.8%; P=0.04). The hypertriglyceridemia
proportion increased with BMI; 16.3% in normal-weight, 33.3% in
overweight (P=0.04) and 56.3% in obese (P=0.005)
categories. Similarly the prevalence of hypertension increased with BMI;
8.2% among normal-weight, 14.3% in overweight (P=0.03) and 12.5%
in obese (P=0.04) categories. Proportion of children with low HDL
level and hypertension was higher with higher severity of fatty liver (Table
I). Higher BMI (OR 35.9; 95% CI 28.5-41.2), severe fatty liver
disease (OR 1.7; 95% CI 1.32-1.96) and female sex (OR 1.9; 95% CI
1.4-2.24) had an association with metabolic syndrome.
Discussion
This study documented fatty liver on ultrasound in
22.4% of selected children (45.6% in overweight children, 18.9% in
normal-weight children). Presence of fatty liver increased with rise in
BMI, and derangement in liver enzymes increased with fatty liver
severity and BMI. Low HDL and hypertriglyceridemia were the commonest
metabolic derangements observed.
Exclusion of under-weight children, including only
private school students, and non-availability of liver biopsy findings
for confirmation of NAFLD are some of the limitations of the present
study.
Reports on NAFLD among Indian children are limited.
Among 4-18 years old Kashmiri school children, NAFLD was documented in
7.4% in all children (26% in obese children) [19]. In a hospital-based
study from Delhi, 3% of children aged 5-12 years had fatty liver [20].
NAFLD was documented on ultrasound in 33.9% normal-weight and 66.1% of
overweight adolescents aged 11-15 years in Mumbai [21]. We documented
higher proportion of NAFLD among normal-weight children, but the
proportion in over-weight/obese children was comparable. Among Iranian
children with NAFLD, raised liver enzymes were reported in 4.1% and 6.6%
of the normal-weight children and 16.9% and 14.9% of the obese children
(6-18 years), respectively [11]. Although higher proportion of liver
enzyme derangement was observed in our study, the trend across the BMI
category and severity of fatty liver was consistent. Proportion of
metabolic syndrome with NAFLD was comparable to reports among children
from China (32%), Japan (30%) and North America (26%) [22-24].
In conclusion, the present observation indicates high
prevalence of fatty liver in normal-weight and overweight children aged
5-10 years. This early onset of NAFLD in childhood points towards rising
prevalence coupled with increasing overweight/obesity, although the
carry-over effect of transition from underweight to normal-weight or
overweight cannot be ruled out. The current practice of NAFLD screening
based on high BMI is likely to miss a sizable number of normal-weight
children with NAFLD, who are also at risk for progression. Further
research is needed to address the biological basis and associations with
various risk factors and potential interventions to prevent and/or
reverse NAFLD. Additionally, lack of appropriate cut-offs for various
metabolic parameters in metabolic syndrome in Indian children makes
comparison and trend analysis challenging. With high burden of
undernutrition, the prevalence of fatty liver among children related to
overweight and metabolic syndrome may be different from the published
reports.
Contributors: All authors have contributed,
designed and approved the study.
Funding: Indian Council of Medical
Research (NCD/ADHOC/93/2010-11). Competing interest: None.
|
What is Already Known?
•
Prevalence of fatty liver and NAFLD is high in children with
higher BMI (overweight or obese).
What This Study Adds?
•
A high proportion of
normal-weight children also have NAFLD and metabolic
derangements.
|
References
1. Bellentani S, Marino M. Epidemiology and natural
history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol.
2009;8:S4-S8.
2. Schwimmer JB, Deutsch R, Kahen T, Lavine JE,
Stanley C, Behling C. Prevalence of fatty liver in children and
adolescents. Pediatrics. 2006;118:1388-93.
3. Wieckowska A, Feldstein AE. Diagnosis of
nonalcoholic fatty liver disease: invasive versus non-invasive. Semin
Liver Dis. 2008;28:386-95.
4. Amarapurkar D N, Hashimoto E, Lesmana LA, SoIlano
JD, Chen PJ, Goh KL, Asia-Pacific Working Party on NAFLD. How common is
non-alcoholic fatty liver disease in the Asia-Pacific region and are
there local differences? J Gastroenterol Hepatol. 2007;22:788-93.
5. Duseja A. Nonalcoholic fatty liver disease in
India- a lot done, yet more required! Indian J Gastroenterol.
2010;29:217-25.
6. Bajaj S, Nigam P, Luthra A, Pandey RM, Kondal D,
Bhatt SP, et al. A case-control study on insulin resistance,
metabolic co-variates & prediction score in non-alcoholic fatty liver
disease. Indian J Med Res. 2009;129:285-92.
7. Jou J, Choi SS, Diehl AM. Mechanisms of disease
progression in nonalcoholic fatty liver disease. Semin Liver Dis.
2008;28:370-9.
8. Hesham AK. Nonalcoholic fatty liver disease in
children living in the obeseogenic society. World J Pediatr.
2009;5:245-54.
9. Widhalm K, Ghods E, Nonalcoholic fatty liver
diseases: a challenge for pediatricians. Int J Obes (Lond).
2010;34:1451-67.
10. Anderson EL, Howe LD, Jones HE, Higgins JPT,
Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease
in children and adolescents: a systematic review and meta-analysis.
PLoSONE. 2015;10:e0140908.
11. Kelishadi R, Cook SR, Adibi A, Faghihimani Z,
Ghatrehsamani S, Beihaghi A, et al. Association of the components
of the metabolic syndrome with non- alcoholic fatty liver disease among
normal-weight, overweight and obese children and adolescents. Diabetol
Metab Syndr. 2009;1:29.
12. Fu JF, Shi HB, Liu LR, Jiang P, Liang L, Wang CL.
Non-alcoholic fatty liver disease: An early mediator predicting
metabolic syndrome in obese children? World J Gastroenterol.
2011;17:735-42.
13. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH.
Establishing a standard definition for child overweight and obesity
worldwide: international survey. BMJ. 2000;320:1240-3.
14. Cole TJ, Flegal KM, Nicholls D and Jackson AA.
Body mass index cut offs to define thinness in children and adolescents:
international survey. BMJ. 2007;335:194.
15. Needleman L, Kurtz AB, Rifkin MD, Cooper HS,
Pasto ME, Goldberg BB. Sonography of diffuse benign liver disease:
accuracy of pattern recognition and grading. Am J Roentgenol.
1986;146:1011-5.
16. McCarthy HD, Jarrett KV, Crawley HF. The
development of waist circumference percentile in British children aged
5.0-16.9 y. Eur J Clin Nutr. 2001;55:902-7.
17. Raj M, Sundaram KR, Paul M, Deepa AS, Kumar RK.
Obesity in Indian children: time trends and relationship with
hypertension. Natl Med J India. 2007;20:288-93
18. Ferreria AP, Oliveria CER, Franca NM. Metabolic
syndrome and risk factors for cardiovascular disease in obese children;
the relationship with insulin resistance (HOMA-IR). J Pediatr.
2007;83:21-6.
19. Irshad, Zargar SA, Khan BA, Ahmad B, Saif RU,
Kawoosa A, et al. Ultrasonographic prevalence of non–alcoholic
fatty liver disease (NAFLD) in Kashmir valley school children. Int J Sci
Res. 2013;2:299-301.
20. Chaturvedi K and Vohra P. Non-alcoholic fatty
liver disease in children. Indian Pediatr. 2012;49:757-8.
21. Pawar SV, Zanwar VG, Choksey AS, Mohite AR, Jain
SS, Surude RG, et al. Most overweight and obese Indian children
have nonalcoholic fatty liver disease. Ann Hepatol. 2016;15:853-61.
22. Jun-Fen Fu, Hong-Bo Shi, Li-Rui Liu, Ping Jiang,
Li Liang, Chun-Lin Wang, et al. Non-alcoholic fatty liver
disease: An early mediator predicting metabolic syndrome in obese
children? World J Gastroenterol. 2011;17:735-42.
23. Tominaga K, Fujimoto E, Suzuki K, Hayashi M,
Ichikawa M, Inaba Y. Prevalence of non alcoholic fatty liver disease in
children and relationship to metabolic syndrome, insulin resistance and
waist circumference. Environ Health Prev Med. 2009;14:142-9.
24. Patton HM, Yates K, Arida AU, Behling CA, Huang
TTK, Rosenthal P, et al. Association between metabolic syndrome
and liver histology among children with nonalcoholic fatty liver
disease. Am J Gastroenterol. 2010;105:2093-102.
|
|
|
 |
|

