|
|
|
Indian Pediatr 2016;53: 1105-1106 |
 |
Chemical Pleurodesis with Oxytetracycline in
Congenital Chylothorax
|
|
Alpana Utture, Vinayak Kodur and Jayashree Mondkar
From Department of Neonatology, Lokmanya Tilak
Municipal Medical College & General Hospital, Sion, Mumbai, India
Correspondence to: Dr Alpana Utture, Associate
Professor, Department of Neonatology,
LTMMC and LTMGH, Mumbai, India.
Email: [email protected]
Received: November 21. 2015;
Initial review: March 05, 2016;
Accepted: August 09, 2016.
|
Background: Congenital chylothorax is an accumulation of chyle in
the pleural space that may present in neonatal period with respiratory
distress. Case Characteristics: A 34-week preterm who presented
with massive congenital chylothorax complicated with hydrops fetalis.
Outcome: The neonate was treated successfully by pleurodesis with
Oxytetracycline. Message: Pleurodesis with oxytetracycline seems
to be effective in treatment of congenital chylothorax.
Keywords: Chyle, Ligation, Management, Neonate, Pleural
effusion.
|
|
C
hylothorax may be congenital, and occurs due to
lymphatic malformations like lymphangioma, lymphangiectasia and atresia
of the thoracic duct. Acquired chylothorax occurs due to trauma and
post-cardiac surgery. It is also known to be associated with conditions
that increase intrathoracic pressure, like superior vena cava thrombosis
[1]. As chyle is composed of fats, immune cells and proteins,
chylothorax is also associated with metabolic, nutritional and
immunological morbidities, apart from respiratory problems. When
chylothorax is associated with hydrops, it is a potentially
life-threatening condition. Initial treatment is conservative, and
includes keeping the baby nil-by-mouth (NBM), and administration of
total parenteral nutrition (TPN). Octreotide, a somatostatin analogue,
has shown promising results in the treatment of congenital chylothorax
[2]. However, when medical management fails, pleurodesis or ligation of
thoracic duct is the definitive treatment [3,4].
Case Report
A male newborn with a birthweight of 2.5 kg was born
with hydrops fetalis at 34 weeks of gestation by a cesarean section.
Antenatal ultrasound at 27 weeks showed bilateral pleural effusion with
ascites and polyhydramnios. Mother’s blood group was O Positive and
Indirect Coomb’s test was negative. Fetal echocardiography and
hemoglobin electrophoresis of the parents were normal.
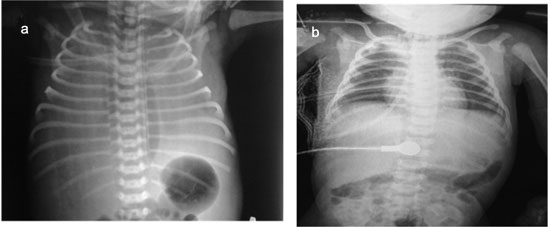 |
|
Fig. 1 Chest X-ray of patient
showing (a) bilateral pleural effusion after birth; (b) after
pleurodesis through the right sided intercostal tube.
|
The neonate had severe respiratory distress, and was
ventilated since birth. Chest X-ray revealed massive bilateral
pleural effusion, and required pleural tapping soon after birth. On day
2, bilateral intercostal drains (ICD) were inserted, because the
effusion was refilling after the initial pleural tap. Initial pleural
fluid was straw-colored, and had a white cell count of 1728 cells/mm3
with 90% lymphocytes and 10% polymorphs. Biochemical analysis showed
glucose 54 mg/dL, total protein 2.8 g/dL, LDH 580 IU/L and triglyceride
98 mg/dL. On day 3, as soon as the baby was fed milk, the pleural fluid
turned milky, and showed a triglyceride level of 171 mg/dL. A diagnosis
of congenital chylothorax was made, and the baby was kept NBM, and
started on TPN. In view of persistent intercostal drainage of chyle, the
baby was started on intravenous Octreotide infusion on day 4 of life at
1µg/kg/hour, which was slowly increased to 7µg/kg/hr. Although the left
ICD output stopped, right ICD output continued to be >100mL/day. In view
of this, on day-20, a decision was made to carry out pleurodesis on the
right side. Oxytetracycline with 2% lignocaine (Oxylab) was administered
into the right pleural cavity at a dose of 20 mg/kg through the ICD, and
the ICD was clamped for 2 hours. Due to failure of adequate response, a
2nd dose was given after 48 hours. The ICD output became nil after two
days. There were no adverse effects noted. Initially, the baby was fed
‘low fat milk’ (containing <0.2% fat per 100 g). Eventually, the baby
was breastfed, and discharged at 2 months of age. On follow up at 6
months of age, the baby was being breastfed, and was gaining weight
adequately. Baby had also attained milestones as per the corrected age.
Discussion
The conservative management of chylothorax is NBM and
prolonged TPN [3]. However, this is associated with morbidities such as
infections, hypoproteinemia and thrombosis. Although intravenous
octreotide has been used in treatment of chylothorax, but not all
effusions respond to it [2]. Pleurodesis or ligation of thoracic duct is
the definitive treatment option [3,4].
Our patient had a massive chylothorax with hydrops
fetalis, which can have a high mortality. The baby was premature, and
needed prolonged mechanical ventilation, including high-frequency
oscillator. Given the respiratory morbidity, the nutritional and
immunological complications, and a lack of response to octreotide, we
decided to perform pleurodesis.
Various agents (Povidone iodine, OK 432,
Tetracycline, Minocycline, Erythromycin and Talc) have been used for
pleurodesis. However, there are no definite guidelines for the agent of
choice [2]. Povidone iodine is known to be associated with renal side
effects [5], and is painful, and intravenous analgesia is needed.
oxytetracycline has been used in adults with malignant chylothorax at a
dose of 20mg/kg [6]. However, there is no previous experience of its use
in neonates.The mechanism of action of oxytetracycline is by initiation
of the inflammatory cascade by mesothelial cells after contact with the
chemical agent. There is activation of the coagulation cascade of the
pleura, fibroblast recruitment, activation, and proliferation, and
collagen deposition [7]. Although, lymphangiography and surgical
ligation are other therapeutic options; due to lack of technical
expertise, these could not be done in our patient. We suggest that
chemical pleurodesis with oxytetracycline is an effective treatment for
congenital chylothorax.
Contributors: AU, VK: were involved in patient
management and preparing the Manuscript. JM: reviewed the manuscript and
approved the final version.
Funding: None; Competing interest:
None stated.
References
1. Soto-Martinez M, Massie J. Chylothorax: Diagnosis
and management in children. Paediatr Respir Rev. 2009;10:199-207.
2. Das A, Shah PS. Octreotide for the treatment of
chylothorax in neonates. Cochrane Database Syst Rev. 2010;9:CD006388.
3. Beghetti M, La Scala G, Belli D, Bugmann P,
Kalangos A, Le Coultre C. Etiology and management of pediatric
chylothorax. J Pediatr. 2000;136:653-58.
4. Merrigan BA, Winter DC, O’Sullivan GC. Chylothorax.
Br J Surg. 1997;84:15-20.
5. O Brissaud, L Desfrere, R Mohsen, MFayon,
JDemarquez. Congenital idiopathic chylothorax in neonates: chemical
pleurodesis with povidone-iodine (Betadine). Arch Dis Child Fetal
Neonatal Ed. 2003;88:F531-3.
6. Walker-Renard PB, Vaughan LM, Sahn SA. Chemical
pleurodesis for malignant pleural effusions. Ann Intern Med.
1994;120:56-64.
7. Antony VB, Rothfuss KJ, Godbey SW, Sparks JA, Hott
JW. Mechanism of tetracycline-hydrochloride-induced pleurodesis:
Tetracycline-hydrochlorid-stimulated mesothelial cells produce a
growth-factor-like activity for fibroblasts. Am Rev Respir
Dis.1992;146:1009-13.
|
|
|
 |
|

