|
|
|
Indian Pediatr 2016;53: 1079-1082 |
 |
Prognostic Value of
Resistive Index in Neonates with Hypoxic Ischemic Encephalopathy
|
|
A Senthil Kumar, Aparna Chandrasekaran, Rajamannar
Asokan and *Kathirvelu Gopinathan
From the Department of Neonatology, CHILDS Trust
Medical Research Foundation and Kanchi Kamakoti CHILDS Trust Hospital,
and *Department of Radiology, Kilpauk Medical College; Chennai, Tamil
Nadu, India.
Correspondence to: Dr Aparna Chandrasekaran,
Department of Neonatology, CHILDS Trust Medical Research Foundation and
Kanchi Kamakoti CHILDS Trust Hospital, Nungambakkam, Chennai 600 034,
Tamil Nadu, India.
Email: [email protected]
Received: February 26, 2016;
Initial review: May 19, 2016;
Accepted: September 02, 2016.
Published online: November 05, 2016.
PII:S097475591600019
|
Objective: To evaluate the role of Resistive index measured by
cranial doppler ultrasonography in predicting the risk of death/
abnormal neurodevelopmental outcomes in term neonates with hypoxic
ischemic encephalopathy. Methods: We enrolled 50 term
asphyxiated neonates with hypoxic ischemic encephalopathy and measured
resistive index within 72 hours from the anterior cerebral artery.
Participants underwent tone and developmental assessment at 6-12 months.
Results: Among the 50 neonates, 25 (50%) had abnormal
resistive index (<0.56 or >0.80). Presence of abnormal resistive index
increased the risk of death/ abnormal neurological outcomes at 6-12
months [RR (95% CI): 7.5 (2.0,8.6), P<0.01]. Conclusion:
An abnormal resistive index is associated with death/ neurodevelopmental
impairment in neonatal hypoxic ischemic encephalopathy.
Keywords: Cranial ultrasound, Mortality, Neurodevelopment,
Outcome.
|
|
A
mong neonates with hypoxic ischemic
encephalopathy (HIE), 15-20% die and nearly 25% develop permanent
neurological deficits [1]. Apgar scores and cord blood acidosis have
been used to predict long-term outcomes of neonates with HIE with
limited usefulness [2-4]. More sensitive techniques like neuroimaging
are limited by cost and expertise [5]. It is therefore, essential to
have evidence-based prognostic tools to inform families regarding
possible long-term sequelae.
Resistive index (RI), calculated from the cerebral
arteries by cranial doppler ultrasonography, reflects cerebral
hemodynamic changes in asphyxia, and has been evaluated as a bedside
marker of risk of subsequent neurodevelopmental impairment in HIE [6].
Studies from high income countries have found decreased cerebral RI to
differentiate asphyxiated neonates from healthy controls and to
reasonably predict the risk of subsequent neurodevelopmental impairment
[7-10]. There is paucity of data on the prognostic role of RI in low-
and middle-income countries. This study was designed to evaluate the
role of abnormal RI measured from anterior cerebral artery in predicting
adverse neurodevelopmental outcomes among term neonates with HIE.
Methods
This prospective cohort study was conducted between
February 2013 and May 2015 at a tertiary-care hospital in India catering
to outborn neonates. We enrolled neonates born at
ł37 weeks gestational
age with (a) birth asphyxia, defined as: Having not cried or
breathed at birth or Apgar score of
Ł5 at 5 minutes of life or need for positive
pressure ventilation for ł1
minute [11]; and (b) evidence of moderate to severe HIE based on
Sarnat and Sarnat’s classification [12]. Neonates with major congenital
anomalies and admitted beyond 72 hours of postnatal age were excluded.
Enrolled neonates received standard respiratory, hemodynamic and
supportive management. No neonate received therapeutic hypothermia as a
treatment modality.
The primary outcome of the study was the risk of
mortality and/or abnormal neurodevelopmental outcomes assessed between
6-12 months age. Death was defined as all-cause mortality occurring
before 12 months of age or last follow up. Abnormal neurodevelopment was
considered as either abnormal tone (assessed using Amiel-Tison’s
method), or ‘suspect’ report on Denver Developmental Screening Test II
(DDST II) performed by a trained developmental pediatrician blinded to
the initial values of RI. Secondary outcomes were to evaluate the
association of abnormal RI with short term morbidities such as death
before discharge, neonatal seizures, shock, respiratory failure and
abnormal electroencephalogram (EEG).
RI was measured for all enrolled neonates within 72
hours of life using pulse wave Doppler ultrasound (General Electric,
Connecticut, United States) with 3.5 MHz transducer, by the principal
investigator, who was trained under a pediatric radiologist for 3
months. Signals were recorded from the anterior cerebral artery (ACA) in
the sagittal plane, keeping the angle of insonation as close to 15 0
as possible. Images were cross-checked by the expert pediatric
radiologist. Resistive index was calculated as RI=(S-D)/S, where S-Peak
systolic velocity, D-End diastolic velocity.
A RI between 0.56 and 0.80 was considered normal
[8,13] and neonates were classified as having either normal or abnormal
RI.
Based on the assumption that 20% and 70% neonates
were likely to die or develop abnormal neurodevelop-mental outcomes in
the normal and abnormal RI groups, respectively [9], with 80% power and
5% alpha error, 19 neonates were needed in each group. Assuming 20%
attrition during follow-up, it was decided to enrol 25 neonates in each
group. The primary outcome was evaluated using chi-square test.
Continuous variables were compared using either Student’s t test or Mann
Whitney U test. Institutional ethics committee approved the study. Data
was analyzed using SPSS version 15.0 and P value <0.05 was
considered statistically significant.
Results
Among 82 term neonates admitted with HIE during the
study period, 50 were included (Fig. 1). Neonates with
normal RI (n=25) were comparable to those with abnormal RI (n=25)
(Table I). Presence of an abnormal RI was associated with
a significantly higher risk of death/ abnormal neurodevelopmental
outcome at 6-12 months (75% (15/20) vs. 10% (2/20); RR (95% CI) =
7.5 (2.0, 8.6), P<0.01). An abnormal RI was also associated with
2.5 times higher risk of death or abnormal neurological examination
before discharge (60% vs. 24%; RR (95% CI) = 2.5 (1.2,5.4), P=0.01),
neonatal seizures as well as abnormal neurosonogram and EEG (Table
II).
TABLE I Baseline Characteristics of Enrolled Population
Baseline variable
|
Neonates with normal RI (n=25) |
Neonates with abnormal RI (n=25) |
|
Gestational age, (weeks), Mean (SD) |
38.8 (0.9) |
38.8 (0.9) |
|
Birth-weight, (g), Mean (SD) |
3288 (337) |
3258 (352) |
|
Male gender, No. (%) |
17 (68) |
18 (72) |
|
Delivered by normal vaginal delivery, No. (%) |
18 (72) |
16 (64) |
|
Small for gestational age*, No. (%) |
3 (12) |
4 (16) |
|
Postnatal age at first evaluation, (h)#; median (IQR)
|
47 (22-68) |
53 (18-67) |
|
Need for resuscitation, No. (%) |
|
|
|
Initial steps |
12 (48) |
3 (12) |
|
Bag and mask ventilation |
9 (36) |
9 (36) |
|
Bag and tube ventilation |
2 (8) |
10 (40) |
|
Chest compressions/ Adrenaline |
2 (8) |
3 (12) |
|
Stage of HIE (Sarnat and Sarnat system), No. (%) |
|
|
|
Moderate |
18 (72) |
16 (64) |
|
Severe |
7 (28) |
9 (36) |
*growth <10th centile for gestational age as per Fenton’s charts [15].
|
TABLE II Association of Resistive Index with Morbidity
|
Outcome variable |
Normal RI |
Abnormal RI |
Relative risk |
P value |
|
(n=25) |
(n=25) |
(95% CI) |
|
|
*Death/ abnormal neurological outcome at 6-12 mo, (n=20) |
2 (10) |
15 (75) |
7.5 (2.0-8.6) |
<0.01 |
|
#Abnormal neurological examination at discharge |
4 (16) |
13 (52) |
3.3 (1.2-8.6) |
<0.01 |
|
Death during hospital stay |
2 (8) |
4 (16) |
2.0 (0.4-9.9) |
0.38 |
|
Death /abnormal neurological examination at discharge |
6 (24) |
15 (60) |
2.5 (1.2-5.4) |
0.01 |
|
Neonatal seizures |
17 (68) |
25 (100) |
1.5 (1.1-1.9) |
<0.01 |
|
Number of Anticonvulsants required to control seizures |
|
|
|
|
|
1 |
15 |
12 |
|
0.13 |
|
>1 |
5 |
12 |
|
|
|
Anticonvulsants for neonatal seizures at discharge
|
1(4) |
13 (52) |
13.0 (1.8-92.0) |
<0.01 |
|
Respiratory failure requiring mechanical ventilation |
10 (40) |
17 (68) |
1.7 (1.0-3.0) |
0.05 |
|
Median (IQR) Duration of ventilation, d |
3 (3-6) |
6 (5-8) |
– |
0.27 |
|
Need for inotropes |
12 (48) |
16 (64) |
1.3 (0.8-2.2) |
0.25 |
|
Inotrope score median (IQR) |
10 (0-30) |
10 (0-40) |
– |
1.00 |
|
Culture positive bacterial sepsis |
5 (20) |
12 (48) |
2.4 (1.0-5.8) |
0.04 |
|
$Abnormal neurosonogram |
4 (16) |
16 (64) |
4.0 (1.6-10.3) |
<0.01 |
|
‡Abnormal EEG |
2 (8) |
12 (48) |
6.0 (1.5-24.1) |
<0.01 |
|
*5 neonates lost to follow up in each group; #Abnormal
neurological examination before discharge as assessed by Amiel
Tison’s method; $Abnormal neurosonogram was defined
as basal ganglia hyperechogenecity, increased periventricular
echogenecity and prominent thalamostriate vessels, ‡Abnormal
electroencephalogram (EEG) was defined as discontinuous
background, burst suppression pattern or seizures. |
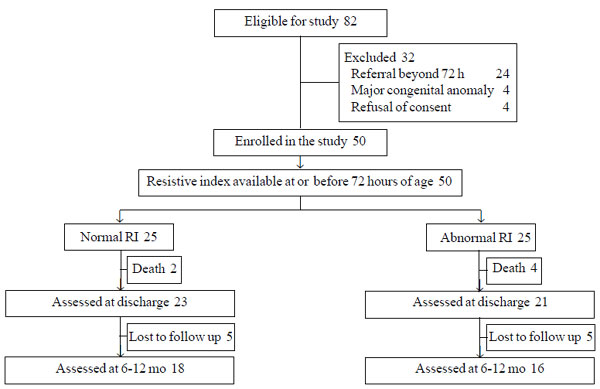 |
|
Fig. 1 Study flow.
|
The sensitivity, specificity, positive predictive
value, negative predictive value and positive likelihood ratio of
abnormal RI to detect the composite outcome of death or abnormal
neurological outcome was 88%, 78%, 75%, 90% and 4.06, respectively.
Discussion
In the present study, we observed that having an
abnormal RI within 72 hours increased the risk of death or abnormal
neurodevelopment at 6-12 months among term neonates with HIE. Short term
morbidities such as abnormal neurological examination at discharge,
seizures, abnormal neurosonogram and EEG were also higher among neonates
with abnormal RI, although the study was not powered to determine these
outcomes.
Loss of cerebral autoregulation in HIE can predispose
to reduced/absent diastolic blood flow in cerebral arteries leading to
increased RI (>0.80) or elevated diastolic flow due to arterial
vasodilation resulting in reduced RI [10,14]. Decreased RI has been well
documented in asphyxia and found to increase the risk of death or
cerebral palsy by 23.4 times [7,8]. The negative predictive value (NPV)
of RI was 90%, implying that finding a normal RI (0.56-0.80) within the
first 72 hours in a neonate with HIE conferred 90% probability that the
neonate will be subsequently normal. This was higher than the NPV of
decreased RI in the study by Jongeling, et al. [8].
RI within 24 hours of age, could not be obtained. We
acknowledge that formal developmental assessment such as Bayley Scales
of Infant Development II (BSID-II) was desirable for identifying
abnormal neurodevelopment.
Considering the modest prognostic potential of RI in
neonates with HIE, it is desirable that neonatologists get familiar with
the optimal usage of this imaging modality, especially in settings
lacking sophisticated neuroimaging techniques. We need more studies
evaluating the impact of neuroprotective strategies, especially
therapeutic hypothermia on RI and its diagnostic accuracy.
Contributors: SK: designed the study protocol,
recruited the participants, performed doppler ultrasonography, and
drafted the initial manuscript; AC: supervised data collection, analyzed
the data and revised the manuscript; RA: helped in designing the study,
data collection, and critically reviewed the final manuscript; KG: study
supervision and manuscript review. All authors approved the final
manuscript
Funding: None; Competing interest:
None stated.
|
What This Study Adds?
• Presence of an abnormally low (<0.56) or
high (>0.80) RI within 72 hours significantly increases the risk
of developing death or abnormal neurodevelopment at 6-12 months
among term neonates with hypoxic ischemic encephalopathy.
|
References
1. Spitzmiller RE, Phillips T, Meinzen-Derr J, Hoath
SB. Amplitude-integrated EEG is useful in predicting neurodevelopmental
outcome in full-term infants with hypoxic-ischemic encephalopathy: a
meta-analysis. J Child Neurol. 2007;22:1069-78.
2. Lie KK, Grřholt EK, Eskild A. Association of
cerebral palsy with Apgar score in low and normal birthweight infants:
population based cohort study. BMJ. 2010;341:c4990.
3. Low JA, Lindsay BG, Derrick EJ. Threshold of
metabolic acidosis associated with newborn complications. Am J Obstet
Gynecol. 1997;177:1391-4.
4. Shah PS, Beyene J, To T, Ohlsson A, Perlman M.
Postasphyxial hypoxic-ischemic encephalopathy in neonates: Outcome
prediction rule within 4 hours of birth. Arch Pediatr Adolesc Med.
2006;160:729-36.
5. Thayyil S, Chandrasekaran M, Taylor A, Bainbridge
A, Cady EB, Chong WKK, et al. Cerebral magnetic resonance
biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics.
2010;125:e382-95.
6. Archer LN, Levene MI, Evans DH. Cerebral artery
Doppler ultrasonography for prediction of outcome after perinatal
asphyxia. Lancet. 1986;2:1116-8.
7. Pinto P, Tekes A, Singhi S, Northington F,
Parkinson C, Huisman T. White–gray matter echogenicity ratio and
resistive index: sonographic bedside markers of cerebral
hypoxic–ischemic injury/edema? J Perinatol. 2012;32: 448-53.
8. Jongeling BR, Badawi N, Kurinczuk JJ, Thonell S,
Watson L, Dixon G, et al. Cranial ultrasound as a predictor of
outcome in term newborn encephalopathy. Pediatr Neurol. 2002;26:37-42.
9. Eken P, Toet MC, Groenendaal F, de Vries LS.
Predictive value of early neuroimaging, pulsed Doppler and
neurophysiology in full term infants with hypoxic-ischaemic
encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1995;73:F75-80.
10. Kudrevičienë A, Basevičius A, Lukođevičius S,
Laurynaitienë J, Marmienë V, Nedzelskienë I, et al. The value of
ultrasonography and Doppler sonography in prognosticating long-term
outcomes among full-term newborns with perinatal asphyxia. Medicina
(Kaunas). 2014;50:100-10.
11. Thomas N, George KC, Sridhar S, Kumar M,
Kuruvilla KA, Jana AK. Whole body cooling in newborn infants with
perinatal asphyxial encephalopathy in a low resource setting: a
feasibility trial. Indian Pediatr. 2011;48:445-51.
12. Sarnat HB, Sarnat MS. Neonatal encephalopathy
following fetal distress. A clinical and electroencephalo-graphic study.
Arch Neurol. 1976;33:696-705.
13. Zamora C, Tekes A, Alqahtani E, Kalayci OT,
Northington F, Huisman TA. Variability of resistive indices in the
anterior cerebral artery during fontanel compression in preterm and term
neonates measured by transcranial duplex sonography. J Perinatol.
2014;34:306-10.
14. Liu J, Cao HY, Huang XH, Wang Q. The pattern and
early diagnostic value of Doppler ultrasound for neonatal
hypoxic-ischemic encephalopathy. J Trop Pediatr. 2007;53:351-4.
15. Fenton TR, Kim JH. A systematic review and meta-analysis to
revise the Fenton growth chart for preterm infants. BMC Pediatr.
2013;13:59.
|
|
|
 |
|

