Sclerosing angiomatoid nodular transformation
(SANT) of the spleen is a rare benign vascular lesion. Since the first
description of this disease by Martel, et al. [1] in 2004,
several more cases have been described. Most cases of SANT have been
incidentally found in adults who were either completely asymptomatic or
had vague abdominal complaints [2]. We searched the MEDLINE database and
reviewed articles related to SANT published in English between January
1, 2004 and July 31, 2012. Reports of SANT in the pediatric age group
are rare, with only three cases reported to date and the youngest case
in an 11-year-old patient [3,4]. Herein, we present the pathological and
imaging evidence used to diagnose SANT in a 3-year-old child.
Case Report
A 3-year-old boy was admitted to our hospital with
complaints of abdominal pain and vomiting 2 hours after being involved
in a car accident. On examination, his vital signs were within normal
limits. Abdominal examination revealed upper abdominal tenderness with
mild muscle spasm. His medical history prior to the car accident and
family history were unremarkable.
The patient’s blood tests, including full blood
count, urea and electrolytes, liver and renal functions, were within
normal limits. Plain radiograph of abdomen ruled out perforation Because
the patient had a closed abdominal injury, suspicion of parenchymatous
intra-abdominal organ hemorrhage was maintained and computed tomography
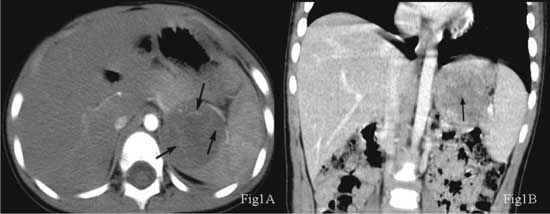
(CT) of the abdomen was performed (Fig. 1). Plain CT
images revealed a circular lesion with low density and a
well-circumscribed border. There was neither a cystic change nor
necrosis or calcification in the lesion. Contrast-enhanced CT scans
indicated an uneven nodular enhancement on the edge of the lesion and
vascular tissue-like bundles from the edge to the enhanced center. In
the portal and delayed phases, the lesion enhanced centripetally with a
wheel-like appearance. In the delay phase, most of the lesions and
spleen parenchyma had similar densities, and a star-like lesion could be
partially seen in the center. Carcinoemboroynic antigen and
a-feto-protein levels
were tested after the detection of tumor mass.
 |
|
Fig. 1. (a) The arterial phase
showed nodular enhancement around the lesion and many enhanced
separations coming from the edge to the center (arrow); (b) The
portal vein phase showed a star-shaped, low-density area (arrow)
in the center of the mass, which had the appearance of a wheel
with spokes.
|
The patient underwent exploratory surgery with
partial splenectomy including total excision of the tumor mass. Gross
examination revealed a well-demarcated, solitary lesion, measuring 4×5×3
cm. The solitary lesion was connected to the spleen without a capsule
and was located on the surface of the spleen with a prominent nodular
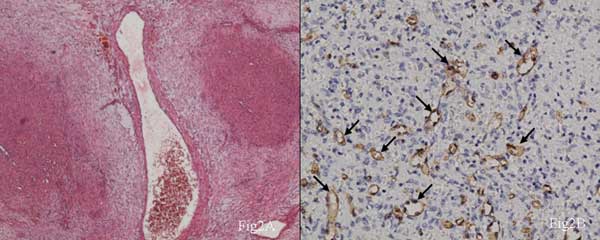
and lobulated shape (Fig. 2a). The tumor mass was chiefly
composed of connective tissue.
 |
|
Fig. 2. (a) The fibrous tissue
was loose, including thick-walled blood vessels, but lacked
prominent collagen staining (HE staining, ×200); (b) Angiomatoid
modules were CD34 positive (HE, ×400).
|
The histopathologic findings revealed that some
fibrous tissue had hyaline or myxoid degeneration and some nodules had
merged to form irregular patterns. The thick-walled vessels contained
myxoid areas with infiltration of inflammatory cells. The lumen of the
vessels in the angiomatoid nodules was small and contained small lacuna
and sinusoid structures with vein-like and capillary-like vessels, which
were more prominent in the center of the nodule. Plasma cells,
eosinophilic leukocytes, and lymphocytes were scattered in the fibrous
stroma, primarily infiltrating the junction between the fiber and blood
vessels. Small amounts of hemorrhage and hemosiderosis were present in
the interstitial tissues and nodules (Fig. 2a).
Immuno-histochemical staining of the small blood vessels within the
angiomatoid nodules were positive for CD34 (Fig. 2b) and
CD31, but negative for glucose transporter 1 (GLUT1), Desmin, S100,
neuron-specific enolase (NSE), CD21, anaplastic lymphoma kinase (ALK),
and Epstein-Barr virus (EBV). Smooth muscle actin (SMA)-positive venous
channels were found in between the epithelioid endothelial cells.
Irregular staining of vimentin and SMA was observed in the stroma. Some
cells in the lesion were CD68+ and CD8+, and the Ki-67+ percentage was
<5%. The observed histomorphology and staining profile supported the
diagnosis of SANT.
The patient recovered well without any post-operative
complications during the post-surgical period, and no evidence of
recurrence was observed during 20 months of follow-up.
Disscussion
Martel, et al. [1] named the condition
sclerosing angiomatoid nodular transformation (SANT) and reported 25
cases; SANT has since been gradually accepted as a distinct condition.
The pathogenesis remains unclear. Some people consider it a bleeding
hemangioma, with necrosis, inflammatory pseudotumor nodules, fibrous
tissue collagen, and nodules-like angiomatoid [5]. Immunohistochemical
staining of specimens from our patient was negative for GLUT1,
suggesting SANT to be a vascular malformation rather than a hemangioma.
Some researchers also believe that SANT is inflammatory pseudotumor-like
[4] and related to EBV infection[6]. The differential diagnosis of SANT
includes splenic hamartoma, for which the abnormal structure is mainly
composed of splenic red pulp without fibrous tissue dividing capillaries
into hemangioma sample nodules. Splenic hemangioma, which mainly
consists of capillary or spongy vessels, could appear as focal
infarction, hemorrhage, and fibrosis without hemangioma nodule formation
and be CD34+, CD31+, and CD8–. In 2010, Hou, et al. [5] reported
10 cases. SANT is typically considered more common in female adult
patients than in males. A literature search revealed four cases of SANT
in children (3 boys and 1 girl). Many patients with SANT are
asymptomatic, and usually the condition is identified incidentally
during physical examination [7]. About half of SANT patients present
with abdominal pain or other non-specific symptoms. Abdominal pain and
bloating have been reported in pediatric cases [3,4]. Li, et al.
[8] first reported CT imaging of SANT, showing the tumors were
low-density with calcifications and the condition progressively
enhanced. In the delayed phase, the lesion had the same density as the
adjacent tissue without a clear boundary between the spleen parenchyma.
The differential diagnoses include Gaucher’s disease, splenic hamartoma,
hemangioma, sarcoid, and a low-grade lymphoma and were difficult to rule
out completely before operation.
Asymptomatic patients with benign vascular splenic
lesions can be treated with conservative management, because SANT is
benign and there has been no report of malignant transformation. In
addition, surgical resection or laparoscopic resection of SANT results
in a good prognosis. In all reported cases of SANT, surgical resection
was both diagnostic and therapeutic. No recurrence or metastasis was
observed over a follow-up duration up to 9 years in the 25 cases
reported by Martel, et al. [1]. With follow-up durations of 7
months to 3 years [3,4], all previously reported pediatric patients
remained asymptomatic and healthy. Neither recurrence nor metastasis was
observed in the present case over a follow-up of 20 months.
Pediatricians and pediatric surgeons need to be aware
about the possibility of SANT occurring in asymptomatic pediatric
patients, though rarely.
Contributors: SZ: drafted the manuscript and
performed some clinical studies; WY: contributions to the conception and
design of the work and diagnosis of the disease HX: performed clinical
examinations, especially during the follow-up period; ZW: helped to
diagnose this disease.
Funding: None; Competing interests: None
stated.
References
1. Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B,
Chan JK, Rosai J. Sclerosing angiomatoid nodular transformation (SANT):
Report of 25 cases of a distinctive benign splenic lesion. Am J Surg
Pathol. 2004,28:1268-79.
2. Budzyñski A, Demczuk S, Kumiega B, Migaczewski M,
Mat³ok M, Zub-Pokrowiecka A. Sclerosing angiomatoid nodular
transformation of the spleen treated by laparoscopic partial splenectomy.
Videosurgery and Other Miniinvasive Techniques. 2011;6: 249-55.
3. Bamboat ZM, Masiakos PT. Sclerosing angiomatoid
nodular transformation of the spleen in an adolescent with chronic
abdominal pain. J Pediatr Surg. 2010;45:E13-6.
4. Vyas M , Deshmukh M, Shet T, Jambhekar N. Splenic
angiomatoid nodular transformation in child with inflammatory
pseudotumor-like areas. Indian J Pathol Microbiol. 2011;54:829-31.
5. Hou J, Ji Y, Tan YS, Shi DR, Liu YL, Xu C, et
al. Sclerosing angiomatoid nodulartransformation of spleenÿa
clinicopathologic study of 10 cases with review of literature. Chin J
Pathol. 2010;39:84-7.
6. Zhang MQ, Lennerz JK, Dehner LP. Granulomatous
inflammatory pseudotumor of the spleen: association with Epstein-Barr
virus. Appl Immunohistochem Mol Morphol . 2009;17:259-63.
7. Falk GA, Nooli NP, Morris-Stiff GÿPlesec TP,
Rosenblatt S. Sclerosing Angiomatoid Nodular Transformation (SANT) of
the spleen: Case report and review of the literature. Int J Surg Case
Rep. 2012;3:492-500.
8. Li L, Fisher DA, Stanek AE. Sclerosing angiomatoid
nodular transformation (SANT) of the spleen: addtion of a case with
focal CD68 staining and distinctive CT features. Am J Surg Pathol.
2005;29:839-41.
9. Zeeb LM, Johnson JM, Madsen M S, Keating DP.
Sclerosing angiomatoid nodular transformation. Am J Roentgenol.
2009;192:W236-8.
10. Thacker C, Korn R, Millstine J, Harvin H, Van
Lier Ribbink JA, Gotway MB. Sclerosing angiomatoid nodular
transformation of the spleen: CT,MR,PET, and 99mTc-sulfur colloid SPECT
CT findings with gross and histopathological correlation. Abdom Imaging.
2010;35:683-9.

