|
|
|
Indian Pediatr 2015;52: 1035-1040 |
 |
Nasal Mask Versus Nasal Prongs for
Delivering Nasal Continuous Positive Airway Pressure in Preterm
Infants with Respiratory Distress:
A Randomized Controlled Trial
|
|
Sorabh Goel, Jayashree Mondkar, Harshad Panchal,
Deeparaj Hegde, Alpana Utture and Swati Manerkar
From Department of Neonatology, Lokmanya Tilak
Municipal Medical College and Lokmanya Tilak Municipal and General
Hospital, Mumbai, India.
Correspondence to: Dr Sorabh Goel, Department of
Neonatology, LTMMC and LTMG hospital, Sion (West), Mumbai 400 022,
India.
Email:
[email protected]
Received: April 09, 2015;
Initial review: June 02, 2015;
Accepted: October 13, 2015.
|
Objective: To compare the effectiveness of nasal continuous positive
airway pressure delivered by Nasal mask vs Nasal prongs with
respect to continuous positive airway pressure failure.
Study design: Randomized, controlled, open label,
trial.
Setting: Tertiary care level III neonatal unit.
Participants: 118 preterm infants-gestational age
(27-34 weeks) requiring nasal continuous positive airway pressure as a
primary mode for respiratory distress, who were treated with either
nasal mask (n=61) or nasal prongs (n=57) as interface.
Primary outcome: Need for mechanical ventilation
within 72 h of initiating support.
Results: Nasal continuous positive airway
pressure failure occurred in 8 (13%) of Mask group and 14 (25%) of
Prongs group but was statistically not significant (RR 0.53, 95% CI
0.24-1.17) (P = 0.15). The rate of pulmonary interstitial
emphysema was significantly less in the Mask group (4.9% vs.
17.5%; RR 0.28, 95% CI 0.08-0.96; P = 0.03). Incidence of
moderate nasal trauma (6.5% vs 21%) (P=0.03) and overall
nasal trauma (36% vs 58%) (P=0.02) were significantly lower in
mask group than in the prongs group.
Conclusion: Nasal continuous positive airway
pressure with mask as interface is as effective as prongs but causes
less nasal trauma and pulmonary interstitial emphysema.
Keywords: Management, Mechanical ventilation, Non-invasive
ventilation, Respiratory distress.
|
|
Nasal continuous positive airway pressure (NCPAP)
is a simple, low cost and non-invasive method of ventilating a sick
newborn [1]. Bubble CPAP is the most commonly used modality for delivery
of NCPAP [2,3]. Traditionally, short bi-nasal prongs have remained the
standard of care for delivery of NCPAP. The limitations of delivering
NCPAP with prongs include mechanical difficulties in maintaining the
nasal prongs, poor tolerance of the infant to the apparatus,
difficulties in positioning the neonate, columella injury and septal
deformities [4-6].
Nasal masks are increasing being used for delivering
CPAP in recent times due to their ease of application [7]. A randomized
trial in neonates <31 weeks gestation comparing nasal mask with binasal
prongs showed less intubation rate within 72 hours for the treatment of
respiratory distress syndrome (RDS) or in post-extubation setting with
nasal mask [8]. A recent randomized controlled trial (RCT) from India
reported a 6% reduction in the oxygen requirement at 2 hours of CPAP
initiation with nasal mask as compared to nasal prongs [9]. Nasal trauma
has been reported with the use of both nasal masks and prongs and occurs
equally with each interface [10,11]. There is need for more evidence
before nasal masks can replace short binasal prongs. Our aim was to
compare the effectiveness of these two modes of CPAP delivery in an
Indian scenario using Bubble CPAP.
Methods
This randomized controlled trial was conducted at a
Level III neonatal intensive care unit (NICU). It was conducted from
March 2014 to February 2015, following approval from the Institutional
ethics committee. Infants were eligible for inclusion if they were born
between 27-34 weeks gestation by best obstetric estimate (dated by early
obstetric ultrasound or last menstrual period) and had respiratory
distress at birth. Babies were initially stabilized in the labor room
and then transported to the NICU. Randomization was done post-initial
stabilization if eligibility criteria was met. For the purpose of this
study, respiratory distress at initiation was defined as
Silverman-Anderson score (SAS) of 3-6 with FiO 2
requirement between 21-60% to maintain SpO2
between 90-95%. Babies with 5 minute Apgar scores
£5, those with major
congenital malformation, and those with antenatally diagnosed congenital
heart disease were excluded from the study. Written informed consent was
taken prior to enrolment.
Intervention: Enrolled infants were randomized to
receive either Nasal mask (group 1) or Nasal prongs (group 2) as a mode
of NCAP delivery interface. Randomization was done using a computer
generated randomization chart with sealed opaque, sequentially numbered
envelopes. The physician on call opened sequentially numbered sealed
opaque envelopes and randomized infants to respective groups. Access to
envelopes was restricted to designated physicians. Blinding of
intervention and outcome measurement was not feasible because of the
nature of intervention
Infants in the Mask group were delivered NCPAP using
Fisher and Paykel Infant Nasal Mask in small (BC800), medium (BC801) and
large (BC802) sizes based on best estimate using the nasal mask scale
provided by the company. Masks were connected to Fisher and Paykel
‘Bubble CPAP system’(BC151) using Fisher and Paykel ‘Flexi Trunk Midline
Interface’ (BC191 - 70 mm) and appropriate sized Fisher and Paykel
‘Infant Bonnet’ depending on Head circumference (BC300 - small, BC303 -
medium, BC306 – large). Infants in the Prong group were delivered NCPAP
using appropriate sized Hudson RCI Infant Nasal Prong CPAP cannula
system (size 0 and 1). The prongs were connected to Fisher and Paykel
‘Bubble CPAP system’(BC151) directly using pins and rubber bands over
appropriate sized bonnets provided with the Hudson Nasal prong CPAP
cannula system.
CPAP was initiated at a pressure of 5 cms of H 2O
with FiO2 sufficient to
maintain SpO2 of 90-95%.
CPAP pressure and FiO2 were
titrated to baby’s requirements to a maximum of 60% FiO2
and CPAP of 8 cms H2O. Flows
were adjusted to maintain adequate bubbling, not exceeding 8 litres/min.
Nasal toilet was provided every 4 hourly and the nursing staff evaluated
for nasal trauma daily in each shift. Nasal trauma was classified at
point of CPAP removal as: Mild trauma: erythema/tenderness; Moderate
trauma- excoriation/crusting/bleeding; and Severe trauma- narrowing of
the passage. Repositioning of the interface and external massage was
given for mild nasal trauma. Mupirocin ointment was applied for
moderate/severe trauma to prevent it from further worsening. Weaning
from CPAP was achieved initially by stepwise reduction of FiO2
to 30%, and then subsequently, CPAP was decreased gradually with removal
at 4 cms of water.
Babies in both the groups were administered natural
bovine surfactant (Survanta) at a dose of 100mg/Kg in 4 equal aliquots
by INSURE (Intubation, Surfactant and Extubation) technique if FiO 2
requirement was >30%, as per routine unit protocol. A repeat dose of
surfactant was given if the FiO2
requirement did not come down to <30% after 12 hours of first dose. All
infants enrolled in the study received a loading dose of 10 mg/kg
caffeine base and then 2.5 mg/kg 24 hours after the loading dose and
daily thereafter. The regular dose of caffeine was increased to a
maximum of 5 mg/kg caffeine base daily if the baby had apneic spells on
CPAP. All babies were started on trophic feeds of human milk by 48-72
hours, if hemodynamically stable.
Outcomes: The primary outcome was CPAP failure,
defined as the need for intubation and mechanical ventilation within 72
hours of initiation of respiratory support. Infants were intubated and
ventilated if they met 2 or more of 5 failure criteria, at maximum CPAP
settings of pressure 8 cms and FiO 2
60% viz. (i) worsening clinical signs of respiratory
distress (increasing tachypnea, expiratory grunting, intercostal,
subcostal, and/or sternal recession); (ii) apnea treated with
positive pressure ventilation (PPV) by mask on two or more occasions in
1 hour; (iii) FiO2
>0.6 to maintain SpO2
³90% for >30 minutes;
(iv) pH <7.2 on two arterial blood gases taken >30 minutes apart;
and (v) PCO2>60 mm Hg
on two arterial blood gases taken >30 minutes apart.
The secondary outcomes related to respiratory support
were duration of CPAP support, duration of supplementary oxygen
requirement, maximal flow, PEEP and oxygen requirement, incidence of air
leaks and Broncho-pulmonary dysplasia. Other outcomes included incidence
of patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH)
grades 3 and 4, necrotizing enterocolitis (NEC), retinopathy of
prematurity (ROP) ³stage
3, culture-proven early and late-onset sepsis, time to full feeds,
length of hospital stay, mortality and nasal trauma.
Infants were monitored as per standard nursing
protocols. All babies on NCPAP had a large bore oro-gastric tube placed
open to the atmosphere in vertical position, to avoid distension of the
stomach. Data collection of maternal variables included maternal
complications, mode of delivery and antenatal steroids. Infant variables
included birth weight, gestational age, presence of IUGR (weight <10th
on Lubchenco percentile), need for resuscitation, FiO 2
requirement and SAS score at initiation of NCPAP support. Vital signs,
FiO2 requirement, CPAP
settings, SAS scores and blood gases of the infant were recorded at
regular intervals as per unit policy.
PDA was diagnosed clinically and confirmed by
echocardiography wherever possible. IVH was defined by bed side
sonography using the Papile classification [12]. NEC was classified
according to Bell’s classification, as modified by Kliegman and Walsh as
stage II or greater [13]. ROP was defined according to the international
classification of retinopathy of prematurity [14]. BPD was defined
according to the National Institutes of Health consensus definition
[15]. Full feeds were defined as feeds that reached 150 mL/kg per day
and sustained for 3 consecutive days. All secondary outcomes were
determined before discharge home from hospital unless stated otherwise.
On the basis of NICU data from previous two years
before this study, around 40% of infants with a completed gestational
age <34 weeks who received NCPAP as primary treatment of respiratory
distress required intubation and invasive ventilation in our NICU. The
interface on which infants started was not recorded but was likely to be
prongs in the majority. We hypothesized that using nasal mask would
reduce the need for intubation and mechanicical ventilation to 20% (an
absolute reduction of 20%). With a two sided alpha error of 0.05 and
beta error of 0.2 (power 80%), the estimated sample size was 118 (59 in
each group).
Statistical analyses: Baseline characteristics
and outcome measures on nominal scales were analyzed by chi square test
or Fisher exact test as appropriate. Baseline characteristics and
outcome measures on continuous scales were analyzed by using two sample
t test or Mann Whitney U test as appropriate. Statistical significance
was considered if the P value was <0.05. Statistical analysis was
performed by applying intention to treat principle. SPSS software for
Windows version 18 (IBM SPSS, Inc, Chicago, IL) was used for statistical
analysis.
Results
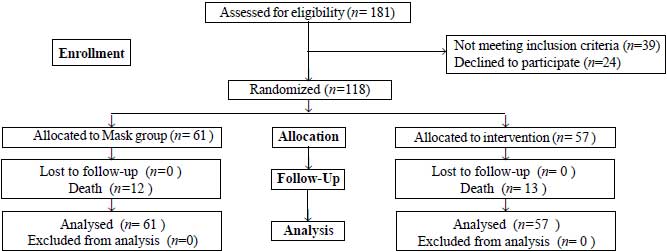
A total of 340 infants born at <34 weeks gestation
were admitted in NICU during the study period, out of which 181 infants
were assessed for inclusion (Fig. 1). 118 infants were
randomly assigned with 61 to Mask and 57 to Prongs group. The
baseline demographic characteristics of enrolled infants were similar
(Table I).
 |
|
Fig. 1 The flow of participants in the
study.
|
TABLE I Baseline Characteristics of the Study Population (N=118)
|
Baseline Characterstics |
Nasal Mask |
Nasal Prongs |
|
(n=61) |
(n=57) |
|
Gestational age (wks) |
30.7 (2.2) |
30.3 (2.2) |
|
Birthweight (g) |
1218 (201) |
1176 (191) |
|
Male, n (%) |
32 (52) |
27 (47) |
|
Vaginal delivery, n (%) |
48 (78) |
40 (70) |
|
IUGR, n (%) |
14 (23) |
10 (17) |
|
PPROM, n (%) |
22 (36) |
17 (30) |
|
Antenatal steroid, n (%) |
36 (59) |
35 (62) |
|
Resuscitation need, n (%) |
28 (46) |
23 (40) |
|
Apgar score 5 min |
9 (8,9) |
8 (7,9) |
|
Age at respiratory support (h) |
1.8 (0.5) |
1.8 (0.4) |
|
Surfactant |
36 (59) |
29 (51) |
|
Multiple doses, n (%) |
12 (20) |
10 (18) |
|
Age (h) |
2.8 (0.8) |
2.8 ( 1.5) |
|
Initial FiO2 (%) |
36.7 (7.1) |
37.2 (8.1) |
|
Maximum FiO2 (%) |
41.0 (8.0) |
42.0 (8.3) |
|
Maximum flow (L/min) |
6.2 (0.7) |
5.9 (0.4) |
|
Maximum CPAP* |
6 (5,6) |
6 (5,7) |
|
All values in mean (SD) unless indiated; *median (interquartile
range); CPAP: Continous positive airway pressure; PPROM: Preterm
premature scripture of membranes; IUGR: Intrauterine growth
retardation. |
CPAP failure was seen in 13% infants on nasal mask
and in 25% infants on nasal prongs, but failed to reach statistical
significance (P = 0.15) (Table II). Incidence of
pulmonary interstitial emphysema was significantly lesser in infants on
nasal mask as compared to nasal prongs (P=0.03), although higher
flows were required in mask group which was statistically significant
[6.2 (0.7) vs 5.9 (0.4) L/min, P= 0.008]. There was a
significant lower incidence of overall nasal trauma in mask group as
compared to prongs (36% vs 58%; RR 0.62, 95% CI 0.41-0.93; P=0.02).
In terms of severity of nasal trauma, the infants in mask group had
lower incidence of moderate trauma as compared to those in prongs group
(7% vs. 21%; RR 0.31, 95% CI 0.10-0.91; P=0.02) which was
statistically significant. The two groups were similar in terms of mild
(P=0.55) and severe (P=0.48) nasal trauma. All the infants
in the study with mild and moderate nasal trauma recovered fully before
hospital discharge except one with columella necrosis who had prolonged
NCPAP support with prongs for 3 weeks.
TABLE II Comparison of Outcomes between Nasal Mask and Nasal Prongs Groups
|
Outcomes |
Nasal Mask (n=61) |
Nasal Prongs (n=57) |
RR (95 % CI) |
P value |
|
NCPAP Failure, n (%) |
8 (13) |
14 (25) |
0.53 (0.24-1.17) |
0.15 |
|
Duration of CPAP (d) * |
6.1 (3.0) |
5.3 (2.3) |
1.15 (0.97-1.37) |
0.09 |
|
Duration of supplementary oxygen (d)# |
5 (3.5,8) |
4 (4,9) |
1.16 (0.32-4.13) |
0.49 |
|
Pulmonary interstitial emphysema, n (%) |
3 (4.9) |
10 (17.5) |
0.28 (0.08-0.96) |
0.03 |
|
Pneumothorax, n (%) |
3 (4.9) |
2 (3.5) |
1.40 (0.24-8.08) |
1.00 |
|
PDA, n (%) |
11 (18) |
14 (24.5) |
0.73 (0.36-1.48) |
0.49 |
|
IVH, grades 3 and 4, n (%) |
3 (4.9) |
7 (12.2) |
0.40 (0.10-1.47) |
0.19 |
|
ROP ³ stage 3, n (%) |
4 (6.5) |
6 (10.5) |
0.62 (0.18-2.09) |
0.51 |
|
BPD, n (%) |
4 (6.5) |
3 (5.2) |
1.24 (0.29-5.32) |
1.00 |
|
NEC, n (%) |
3 (4.9) |
5 (8.7) |
0.56 (0.14-2.23) |
0.48 |
|
EOS, n (%) |
11 (18) |
9 (16) |
1.14 (0.51-2.55) |
0.80 |
|
LOS, n (%) |
15 (25) |
12 (21) |
1.16 (0.59-2.27) |
0.66 |
|
Feeding intolerance, n (%) |
12 (20) |
15 (26) |
0.74 (0.38-1.45) |
0.51 |
|
Time to full feeds (d)* |
13.9 (3.3) |
14.5 (3.2) |
0.96 (0.88-1.04) |
0.38 |
|
Duration of hospital stay (days)# |
22 (15,27) |
19 (15,28) |
1.08 (0.65-1.77) |
0.95 |
|
Mortality, n (%) |
12 (20) |
13 (23) |
0.86 (0.42-1.73) |
0.48 |
* mean (SD); #median (interquartile range)
BPD: Bronchopulmoary dytsplasia; PDA: Patent ductus arteriosis;
IVH: Intraventricular haemorrhage; ROP: Retinopathy of
prematurity; NEC: Necrotizing enterocolitis; EOS: Early-onset
sepsis (culture proven); LOS: Late-onset sepsis (culture
proven). |
Discussion
In our study, we found a reduced NCPAP failure within
72 hours of initiation of respiratory support in preterms in the mask
group when compared with the prongs group; however, this difference was
statistically not significant. Incidence of PIE was significantly lower
in the Mask group than the Prongs. There was a significantly lower
inci-dence of moderate and overall nasal trauma in Mask group.
The major limitation of our study was its non-blinded
design with potential for bias in particular with assessment of nasal
trauma due to the very nature of the intervention. When we planned the
study, we assumed NCPAP failure rate of 40% in Prongs group and 20% in
Mask group. On completion of our study we found these rates were 25% and
13%, respectively. Our study was therefore underpowered to demonstrate
difference, if any, between the two intervention interfaces. Another
limitation of our study was it being a single center study, as NCPAP
failure rates may vary in other units.
A study by Kerian, et al. [8], compared mask
vs prongs using IFD, and found that in terms of NCPAP failure,
nasal mask was superior than prongs which was statistically significant
(28% vs 52%). Our overall CPAP failure rate was comparable to the
previously done studies [16,17]. Air leaks are known complications of
CPAP therapy [18]. We found low incidence of PIE in the mask group which
was an unexpected finding in our study. Similar findings in the mask
group have been previously reported [8], though not reaching statistical
significance. Paradoxically, we found that delivering NCPAP with nasal
masks required higher flows than prongs which was statistically
significant. We assume that need for higher flows could be due to some
leak at the interface level, which could have played a protective role
for air leaks in the mask group. Further studies are required to
elucidate, if any, the causal relation between the interface and air
leaks.
Nasal trauma has been found to be a major drawback
associated with NCPAP use. Injury pattern in the nasal mask group was
primarily seen at the base of nasal bridge with occasional injuries at
the junction between the nasal septum and the philtrum sparing the
columella and septum. This may be because the mask rests on the nasal
bridge and philtrum with constant pressure hampering local tissue
perfusion which triggers breach of skin barrier leading to inflammation
and nasal trauma. Injury pattern in prong group was mainly seen at
columella and anterior part of nasal septum which may be due to constant
pressure between the two prongs. These findings of different sites of
injury are consistent with the pattern described in a previous study of
nasal trauma [11].
We conclude that NCPAP with mask as interface is
equally effective as providing NCPAP with short bi-nasal prongs, but
causes less PIE and nasal trauma. We have used Bubble CPAP as a primary
mode of respiratory support thus making our results more generalizable
and applicable in resource-limited settings. As masks and prongs cause
nasal trauma in differing distribution, we suggest that the interface to
be alternated after every 72 hours if NCPAP to be used for prolonged
duration. We recommend more multicentric RCTs with appropriate sample
size to evaluate impact of various delivery interfaces on NCPAP success
along with associated side effects.
Acknowledgments: Dean, LTMMC and Hospital,
Mumbai for permission. Dr Nandkishor S Kabra (Associate
professor, Department of Neonatology, Seth G.S. Medical college and KEM
Hospital, Mumbai) for analyzing the data.
Contributors: SG, JM, HP: conceived and designed
the study; JM, AU, SM: were involved in patient care; SG, DGH, HP:
collected the data; SG, HP: analysis and interpretation of data; SG,
DGH, JM: Drafting the manuscript. All authors approved the final
manuscript.
Funding: None; Competing interests:
None stated.
|
What is Already Known?
• Nasal Continuous Positive Airway Pressure
(CPAP) can be delivered by using Nasal mask or Nasal prongs as
an interface in preterm infants with respiratory distress.
What This Study Adds?
• Nasal mask is as effective as prongs for
NCPAP delivery in preterm infants but causes less nasal trauma
and pulmonary interstitial emphysema.
|
References
1. Polin RA, Sahni R. Newer experience with CPAP.
Semin Neonatol. 2002;7: 379-89
2. Pillow JJ, Hillman N, Moss TJ. Bubble continuous
positive airway pressure enhances lung volume and gas exchange in
preterm lambs. Am J Respir Crit Care Med. 2007;176:63-9.
3. Tapia JL, Urzua S, Bancalari A. Randomized trial
of early bubble continuous positive airway pressure for very low birth
weight infants. J Pediatr. 2012;161:75-80
4. Bonner KM, Mainous RO. The nursing care of the
infant receiving bubble CPAP therapy. Adv Neonatal Care. 2008;8:78-95.
5. McCoskey L. Nursing care guidelines for prevention
of nasal breakdown in neonates receiving nasal CPAP. Adv Neonatal Care.
2008;8:116-24.
6. Kattwinkel J, Fleming D, Cha CC, Fanaroff AA,
Klaus MH. A device for administration of continuous positive airway
pressure by the nasal route. Pediatrics. 1973;52:131-4.
7. Kieran EA, Walsh H, O’Donnell CPF. Survey of nasal
continuous positive airways pressure (NCPAP) and nasal intermittent
positive pressure ventilation (NIPPV) use in Irish newborn nurseries.
Arch Dis Child Fetal Neonatal Ed. 2011;96: F156.
8. Kieran EA, Twomey AR, Molloy EJ, Murphy JF,
O’Donnell CP. Randomized trial of prongs or mask for nasal continuous
positive airway pressure in preterm infants. Pediatrics.
2012;130:1170-6.
9. Chandrasekaran A, Sachdeva A, Sankar MJ, Agarwal
R, Deorari AK, Paul VK. Nasal mask versus nasal prongs in the delivery
of continuous positive airway pressure in preterm infants – An open
label randomized controlled trial. E-PAS. 2014:2936:512.
10. Fischer C, Bertelle V, Hohlfeld J, Forcada-Guex
M, Stadelmann-Diaw C, Tolsa JF. Nasal trauma due to continuous positive
airway pressure in neonates. Arch Dis Child Fetal Neonatal Ed.
2010;95:F447-51.
11. Yong SC, Chen SJ, Boo NY. Incidence of nasal
trauma associated with nasal prong versus nasal mask during continuous
positive airway pressure treatment in very low birthweight infants: a
randomised control study. Arch Dis Child Fetal Neonatal Ed.
2005;90:480-83.
12. Papile LA, Burstein J, Burstein R, Koffler H.
Incidence and evolution of subependymal and intraventricular hemorrhage:
a study of infants with birth weights less than 1,500gm. J Pediatr.
1978;92:529-33.
13. Kliegman RM, Walsh MC. Necrotizing enterocolitis:
pathogenesis, classification and spectrum of illness. Curr Probl Pediatr.
1987;17:213-88.
14. International Committee for the Classification of
Retinopathy of Prematurity. The International Classification of
Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991-99.
15. Bancalari E, Jobe AH. Bronchopulmonary dysplasia.
Am J Respir Crit Care Med. 2001;163:1723-9.
16. Urs PS, Khan F, Maiya PP. Bubble CPAP - a primary
respiratory support for respiratory distress syndrome in newborns.
Indian Pediatr. 2009;46:409-11.
17. Koti J, Murki S, Gaddam P, Reddy A, Reddy MD.
Bubble CPAP for respiratory distress syndrome in preterm infants. Indian
Pediatr. 2010 ;47:139-43.
18. Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet
JM, Carlin JB; COIN Trial Investigators.Nasal CPAP or intubation at
birth for very preterm infants. N Engl J Med. 2008;358:700-8.
|
|
|
 |
|

