|
|
|
Indian Pediatr 2014;51:
975-990 |
 |
Consensus Guidelines on Management of
Childhood Convulsive Status Epilepticus
|
|
*Devendra Mishra, #Suvasini
Sharma, @Naveen
Sankhyan, ^Ramesh
Konanki, $Mahesh
Kamate, °Sujata Kanhere and #Satinder
Aneja;
for the ‡Multi-disciplinary
Group on Management of Status Epilepticus in Children in India
From Departments of Pediatrics, *Maulana Azad Medical College and
#Lady Hardinge Medical College, New Delhi; @Division of Pediatric
Neurology, Department of Pediatrics, PGIMER, Chandigarh; ^Rainbow
Hospital for Women and Children, Hyderabad; $Department of Pediatrics,
KLE University’s JN Medical College, Belgaum; °Department of Pediatrics
and Neonatology, KJ Somaiya Medical College, Hospital and Research
Centre, Mumbai; India.
Correspondence to: Prof Satinder Aneja, Convener, Multi-disciplinary
Group on Management of Status Epilepticus in Children in India;
Director-Professor, Department of Pediatrics, Lady Hardinge Medical
College, New Delhi 110 001, India. Email:
anejas52@gmail.com
|
Justification: Status epilepticus has a wide etiological spectrum,
and significant morbidity and mortality. Management using a
pre-determined uniform protocol leads to better outcomes. Multiple
protocols for management of childhood status epilepticus are available,
without much consensus.
Process: A ‘Multi-disciplinary Consensus
Development Workshop on Management of Status Epilepticus in Children in
India’ was organized. The invited experts included Pediatricians,
Pediatric neurologists, Neurologists, Epileptologists, and Pediatric
intensive care specialists from India, with experience in the relevant
field. Experts had previously been divided into focus groups and had
interacted on telephone and e-mail regarding their group
recommendations, and developed consensus on the topic. During the
meeting, each group presented their recommendations, which were
deliberated upon by the house and a consensus was reached on various
issues; the document was finalized after incorporating suggestions of
experts on the draft document.
Objective: To provide consensus guidelines on
evaluation and management of convulsive status epilepticus in children
in India (excluding neonatal and super-refractory status epilepticus).
Recommendations: Each institution should use a
pre-determined protocol for management of status epilepticus;
pre-hospital management and early stabilization is the key to a
satisfactory outcome of status epilepticus. Pharmacotherapy should not
be delayed for any investigations; the initial management should consist
of a parenteral benzodiazepine by any route feasible. Subsequent
management has been detailed. The group also felt the need for more
epidemiological research on status epilepticus from India, and
identified certain research areas for the purpose.
Keywords: Evaluation, Investigations, Multi-disciplinary,
Pharmacotherapy, Seizure, Treatment.
|
|
S
tatus epilepticus (SE) is a life-threatening
emergency that requires prompt recognition and management [1]. Immediate
treatment of status epilepticus is crucial to prevent adverse neurologic
and systemic consequences [2]. Multiple protocols for management of SE
in children are available both internationally [3-5] and from India
[6-8]. It has previously been demonstrated that use of a pre-determined
protocol for management of SE leads to favorable outcomes [9]. A single
protocol for management of SE in children, suitable for use in the
Indian setting, taking in consideration the common etiologies of SE and
the drugs available, is thus the need of the hour.
Process
A ‘Multi-disciplinary Consensus Development Work-shop
on Management of Status Epilepticus in Children in India’ was organized
by the Association of Child Neurology on 17 th
November, 2013 in New Delhi. The invited experts included General
pediatricians, Pediatric neurologists, Neurologists, Epileptologists,
and Pediatric intensive care specialists from all over India with
experience in the relevant field. This group was designated as the
‘Multi-disciplinary Group on Management of Status Epilepticus in
Children in India’ (Annexure I). In addition, consultants
and residents in Pediatrics were invited as observers. Experts had
previously been divided into focus groups, and had interacted on
telephone and e-mail regarding their group recommendations. During the
meeting, each group presented its recommendations, which were
deliberated upon by the house and a consensus reached on various issues.
At the end of the meeting, it was decided to bring out guidelines on
evaluation and management of Status epilepticus in children in India,
and a Writing group designated for the purpose. Due to the lack of
country-specific epidemiologic information and varying levels of care
available at various centers, it was decided not to categorize the
recommendations by either ‘level of evidence’ or ‘strength of
recommendation’ [10]. The draft document was circulated by e-mail among
all experts and suggestions received incorporated; the final document is
presented here. It does not cover the management of neonatal SE and
Super-refractory SE.
Guidelines
A. Definition and Epidemiology
The most widely used definition for SE is "a seizure
lasting more than 30 minutes or recurrent seizures for more than 30
minutes during which the patient does not regain consciousness" [11,12].
More recently, an operational definition has also been suggested for
adults and children older than 5 years [13] (Box 1). If we
consider the duration for which most new-onset seizures in children
last, once a seizure lasts for more than five to ten minutes, it is
unlikely to stop spontaneously within the next few minutes, and
intervention is indicated [14]. The use of the operational definition
allows early treatment (starting at 5-10 min) [15]. However, in view of
most previous studies on SE having been done using the 30-minute
definition, the group suggests that for research purposes, both the
definitions be considered and data provided with respect to both time
durations.
BOX 1 Important Definitions
|
Status epilepticus (SE): A seizure lasting more than 30 minutes
or recurrent seizures for more than 30 minutes during which the
patient does not regain consciousness .
*Operational definition: Generalized, convulsive status
epilepticus in adults and older children (>5 years old) refers
to >5 min of (i) continuous seizures or (ii) two or more
discrete seizures between which there is incomplete recovery of
consciousness [13].
Refractory SE: Seizures persist despite the administration of
two appropriate anticonvulsants at acceptable doses, with a
minimum duration of status of 60 minutes (by history or on
observation).
Super-refractory SE: SE that continues 24 hours or more after
the onset of anesthesia, including those cases in which the
status epilepticus recurs on the reduction or withdrawal of
anesthesia.
*For the purpose of initiating management. |
SE in children is commonly due to cryptogenic or
remote symptomatic causes in older children, and febrile or acute
symptomatic cause in younger children [9,16]. Majority of childhood
convulsive SE in a UK study (56%) occurred in previously neurologically
healthy children, a quarter of SE were prolonged febrile seizures, and
17% were acute symptomatic [17]. Epidemiological data on SE in India is
limited to a few single-center studies [18-20], with only one providing
exclusive pediatric data [18]. The high proportion of acute symptomatic
etiology, delayed presentation and poor outcome are the commonly
reported findings. In an Indian pediatric intensive care unit (PICU)
study over seven years, 53% had SE as their first seizure and only 60%
had received any treatment prior to coming to the PICU [18]. A recent
multi-centric study on SE in children across nine centers in India also
reported similar findings: 82% acute symptomatic, <3% pre-hospital
treatment, <20% deficit-free survival, and no uniform management
protocol [unpublished data].
B. Pre-hospital Management
Treatment of SE needs to be initiated as early as
possible since once seizures persist for 5 to 10 minutes, they are
unlikely to stop on their own in the subsequent few minutes [21].
Moreover, the longer an episode of SE continues, the more refractory to
treatment it becomes and the greater is the likelihood of complications
[22]. Thus, the need for early treatment, preferably pre-hospital, is
clear.
Pre-hospital management includes both first-aid
during seizures, and pharmacotherapy. The initial care of a child with
convulsions/coma is adequately described in Facility-based Integrated
Management of Neonatal and Childhood Illnesses (F-IMNCI) guidelines of
the Government of India [23] and will not be elucidated here further.
Decision about pharmacotherapy must consider the drug and also the route
of drug delivery (Box 2).
Box 2 Recommendations for Out-of-Hospital Management of
Seizures
|
|
Guiding Principles
|
|
•
Acute treatment with anticonvulsants should be commenced after
continuous seizures or serial seizures >5 min in an
out-of-hospital setting, and efforts made to transfer the
patient to the nearest health care facility.
|
|
•
Prolonged seizures should be treated with either nasal or buccal
midazolam or rectal diazepam when intravenous line is not
available or in the community setting.
|
|
•
Rectal diazepam is safe and effective as first-line treatment of
prolonged seizures in community setting or when intravenous
access is not available.
|
|
•
Buccal or intranasal midazolam is as effective as rectal
diazepam and can be considered as a preferable alternative in
community setting.
|
|
At
Home: Parents
|
|
•
First aid
|
|
•
Rectal diazepam OR buccal midazolam OR intranasal midazolam
|
|
•
Inform doctor/shift to hospital if >5 min (or if more than 2 min
longer than previous seizure duration)
|
|
At
Home/Out of Hospital by Paramedics
|
|
•
First aid - Airway, breathing, circulation, oxygen
|
|
•
Supportive care
|
|
•
Intranasal midazolam OR buccal midazolam OR rectal diazepam
|
|
•
Shift to hospital
|
|
First-level Health Facility (Clinic/PHC/Nursing home)
|
|
•
ABC, Oxygen
|
|
•
Intravenous access feasible:
|
|
– Intravenous lorazepam
(if refrigeration & electric supply), diazepam, or midazolam
|
|
•
Intravenous access not feasible:
|
|
– Intramuscular
injection can be given: IM midazolam
|
|
– Intramuscular
injection not feasible: Intranasal/buccal midazolam, rectal
diazepam
|
| • Shift to higher
center, if required |
Benzodiazepines are the drugs that are currently in
use for pre-hospital therapy for SE and include diazepam, lorazepam and
midazolam. Pre-hospital treatment with benzodiazepines has been shown to
reduce seizure activity significantly compared with seizures that remain
untreated until the patient reaches the emergency department [24]. The
various routes employed include per-rectal (diazepam, lorazepam,
paraldehyde), intranasal (midazolam), buccal (midazolam, lorazepam) and
intramuscular (midazolam).
Rectal diazepam is an approved out-of-hospital
treatment for acute repetitive seizures in children. Response rates have
been demonstrated to be similar to intravenous diazepam [25]. Multiple
randomized, double-blind, placebo controlled studies have demonstrated
that rectal diazepam given by caregivers at home is an effective and
safe treatment for acute recurrent seizures [26-28]. Rectal
administration may be difficult with wheel-chair users and larger
patients. It can be socially unacceptable, and there is increasing
concern about risk of sexual abuse allegations [29,30]. Therefore,
non-rectal routes are gradually gaining favor for use by
relatives/health workers in out-of-hospital settings. Rectal diazepam is
the recommended drug for control of seizures in the F-IMNCI guidelines,
in situations where intravenous access is not available [23].
In the past decade, research evidence has shown that
buccal midazolam is more than or equally effective to rectal diazepam
for children presenting to hospital with acute seizures, and is not
associated with an increased incidence of respiratory depression
[29,31,32]. Therefore, it may be considered as an acceptable alternative
to rectal diazepam [30]. Intranasal midazolam has been shown to be as
effective as intravenous diazepam in the treatment of prolonged febrile
convulsions [4,33-35], and may also be an alternative. More recent data
from the RAMPART study [36] of pre-hospital management of SE in children
and adults has shown intramuscular midazolam to be as safe and effective
as intravenous lorazepam for pre-hospital seizure cessation. This may
therefore emerge to be the agent of choice for out-of-hospital
management of seizure by trained personnel.
Rectal and intranasal lorazepam have also shown
efficacy for termination of acute convulsive seizures in children
[4,37]. However, non-availability of a commercial preparation in India
precludes any firm guidance on non-parenteral use of lorazepam in India.
Parents of all children at risk of seizure recurrence
should be counseled for appropriate home management for seizure [38].
C. Supportive Care and Stabilization
Although convulsive seizures are the most obvious
manifestation, SE is in fact a multisystem phenomenon i.e. prolonged and
ongoing SE affects multiple organ systems. Hence, apart from attempts to
rapidly control seizures, important goals of therapy are
neuro-protection, and prevention and treatment of systemic complications
associated with intravenous AEDs, anesthetic drugs and prolonged
unconsciousness [39]. The supportive care should be tailored to the
health care setting, the clinical presentations of SE, degree of
encephalopathy, and degree of impairment of vital functions.
Airway, Breathing and Circulation
Assessment and care of vital functions is essential
at all stages of managing any child with SE [40]. Adequate care of
airway, breathing and circulaion takes precedence over any
pharmacological therapy.
Airway: It is essential to maintain a patent
airway during all stages of management of SE.
• In all children with brief seizures and altered
sensorium, clearing the oral secretions (mouth, followed by nose)
and keeping the child in recovery position is advisable to prevent
aspiration. Cervical spine should be immobilized if trauma is
suspected.
• In more severe degrees of altered sensorium,
use an oral airway to prevent tongue from falling back.
• Endotracheal intubation in children whose
airway is not maintainable with above measures.
• The airway compromise may occur at any stage;
either as complication of prolonged or ongoing seizure, or due to
respiratory depressant effect of medications.
Breathing: Hypoxemia may result from respiratory
depression/apnea, aspiration, airway obstruction, and neurogenic
pulmonary edema [41].
• All children with SE should have their
breathing and SpO2 monitored continuously.
• All children with ongoing seizures should be
given supplemental oxygen to ameliorate cerebral hypoxia, as it has
been seen that the degree of hypoxia is often underestimated.
• Depending on the duration of SE and degree of
altered sensorium, maintain oxygen saturation by: supplemental
oxygen, AMBU bag, non-invasive continuous positive airway pressure
(CPAP), and invasive ventilation by endotracheal intubation.
Mechanical ventilation may also become necessary when children are
started on continuous infusions of anesthetic agents.
Circulation: Continuous monitoring of
pulse, blood pressure and perfusion should be done in all SE patients.
• Ensure good venous access (preferably have at
least two venous lines); draw necessary blood samples, and start
fluids and anti-epileptic drugs as necessary.
• Maintain blood pressure in the normal range
with necessary measures including: intravenous fluids, fluid
boluses, and inotropes. Invasive blood pressure monitoring should be
considered, if feasible, in children with hypotension and poor
peripheral perfusion either spontaneously or following infusion of
continuous anesthetic agents.
• The choice of IV fluids depends on the
metabolic and glycemic status. If there is hyperglycemia (especially
initial phase of catecholamine excess) it is preferable to give
either dextrose normal saline (DNS) or normal saline. However, in
general, hypotonic fluid should be avoided for initial
resuscitation.
Precipitating Factors and Ongoing Complications
The treating team should anticipate one or more of
the below mentioned problems depending on the duration of SE, age,
underlying etiology, and the associated systemic co-morbidities. The
cerebral and systemic metabolism undergoes changes described as initial
phase of ‘compensation’, and if SE is sufficiently prolonged, later
phase of ‘decompensation’ [42,43]. During the initial phase, prolonged
seizures result in increased cerebral blood flow and metabolism,
excessive catecholaminergic activity and cardiovascular changes. These
in turn result in hyperglycemia, hyperpyrexia, tachycardia, sweating,
hypertension, incontinence, cardiac arrhythmias, and lactic acidosis
[43-46]. If the SE is prolonged, the cerebral autoregulation
progressively fails and cerebral perfusion becomes dependent on systemic
blood pressure resulting in hypoxia, cerebral ischemia, hypoglycemia,
and lactic acidosis [42,43]. Management of these conditions is detailed
in Web Table I. Both hypernatremia (serum
sodium >145 meq/L) and hyponatremia (<135 meq/L) are deleterious for the
brain. The major risks associated with hypernatremia are intracranial
hemorrhage (subdural, subarachnoid and intraparenchymal) and osmotic
demyelination (pontine or extra-pontine) with rapid correction.
Risk of infections is greatly increased in those with
SE, especially when the duration is prolonged. Ventilator-associated
pneumonia, urinary tract infection, pseudomembranous colitis, oral
candidiasis, and speticemia are the common infections [47,48]. Commonest
organisms are P. aeruginosa, A. spp, K. pneumoniae, and Entero
bacteriaceae [48]. Hyperpyrexia, rhabdomyolysis, and raised intracranial
pressure are the other common accompaniments [43,49,50]. Rarely, SE is
associated with ictal bradycardia, stress cardiomyopathy, neurogenic
pulmonary edema, rhabdomyolysis and related renal failure, or bone
fractures [46]. Hypotension is common due to prolonged seizures, IV
benzo-diazepines, or anesthetic agent infusions, and stress
cardiomyopathy (Takotsubo cardiomyopathy) [47,51-53]. Cardiac
arrhythmias are also common (up to 58%), with higher mortality in these
patients [54,55]. Management depends on the cause of hypotension,
hypovolemic shock, distributive shock, cardiomyopathy, or cardiac
arrhythmias (WebTable I).
Although in most cases it is mild, early
identification and aggressive treatment of rhabdomyolysis prevents
complications like renal failure and compartment syndrome. The initial
fluids for resuscitation may include normal saline or 5% dextrose in
water (approximately 2-3 times the daily maintenance). Sodium
bicarbonate may be added to IV fluids, especially if there is associated
metabolic acidosis and/or hyperkalemia [56,57].
D. Investigations
The clinical scenario, including the history and
physical examination, is the most important factor guiding the specific
evaluation that each child will require [58]. The investigations usually
considered include blood chemistries, complete blood count,
antiepileptic drug (AED) levels, toxicological studies, lumbar puncture,
electroencephalography, and neuroimaging (Computed tomography [CT] scan
and Magnetic resonance imaging [MRI]). The major part of evaluation can
be performed after the child has been stabilized in an intensive care
setting, and the seizures have been completely or partially controlled
[58,59].
The investigations done are primarily to (i)
determine the cause of status epilepticus, (ii) to look for
complications of status epilepticus per se, and (iii) to
identify the side-effects of drugs. Early identification of the etiology
can result in aggressive specific management of cause. The
investigations may vary depending on whether it is the first episode of
SE in a normal child, or SE in a child with pre-existing epilepsy and
already receiving AEDs [16,58]. The tests are detailed below (in no
specific order), and listed in Table I in the order of
importance.
TABLE I Investigations in a Child With Status Epilepticus
|
First Line |
Second Line* |
|
SE in a child without history seizures |
|
Ionic/total calcium (especially <2yr) |
MRI |
|
Random blood sugar
|
EEG |
|
Sodium (especially <6mo) |
If clinical suspicion: Urine toxicology |
|
Add, if febrile: Complete blood count; Lumbar puncture# |
|
|
SE in known epilepsy patient
|
|
• Known non-compliance/Missed dose/Recent drug or dose changes |
|
|
Anti-epileptic drug level |
Random blood sugar
|
|
Consider, if febrile |
Ionic/total calcium (especially <2y) |
|
Complete blood count |
Sodium (especially <6mo) |
|
Lumbar puncture# |
If clinical suspicion: Urine toxicology |
|
• No known precipitating event |
|
|
Ionic/total calcium (especially <2y) |
If clinical suspicion: Urine toxicology |
|
Random blood sugar |
Anti-epileptic drug level (if feasible) |
|
Sodium (especially <6mo) |
|
|
Add, if febrile: Complete blood count; Lumbar puncture# |
|
If refractory SE or Persistent encephalopathy: Video-EEG
monitoring |
|
SE: Status epilepticus; *EEG and Neuroimaging should be done
later, after stabilization of the patient; #A central nervous
system infection may be considered even in afebrile infants (<6
mo) and lumbar puncture done, based on clinical setting. |
Blood Chemistries
Electrolyte and glucose abnormalities have been
reported to be present in 1-16% of children with SE, although it is
unclear whether they were the etiology in all and did treatment lead to
cessation of the SE [16].
Serum calcium: Hypocalcemia as a cause of
seizures is common in our country [60,61], usually due to vitamin D
deficiency, and presents as a cluster of seizures in infancy. Early
recognition avoids unnecessary treatment with AEDs and other
interventions. Ionic calcium is more reliable as a guide for treatment
and levels are usually <0.8 µmol/L in symptomatic children. However, all
children with SE and subnormal ionized calcium levels (<1.2 µmol/L)
should be treated. Total serum calcium, if done, should always be
combined with estimation of phosphorous, serum alkaline phosphatase and
serum albumin, for proper interpretation. Serum calcium estimation is an
essential investigation for all children younger than 2 years with
status epilepticus, irrespective of presence or absence of suggestive
features.
Random blood sugar: Should be done in all
children at presentation (especially in children less than 5 years)
[58], as hypoglycemia may be responsible for seizures, and both hypo- or
hyper-glycemia cause brain damage. When hypoglycemia is documented,
urine ketones and reducing sugar should also be evaluated.
Serum sodium: Hyponatremia has been
reported to be a cause in 1% of new-onset childhood convulsive SE [62].
However, most children with this abnormality were found to have
suggestive features on history and clinical examination [63]. As this
finding has important therapeutic implications [58], serum sodium
estimation should be done in all, if feasible.
Metabolic disorders: Metabolic disorders are
reportedly present in around 4.2% of children with SE [16], though their
etiological significance is unclear. Routine metabolic workup therefore
appears unwarranted. However, arterial blood gas estimation should be
done in all children with established SE, if facilities are available;
or when transferring to the Intensive Care Unit (ICU).
Workup for Infections
Blood counts: May be done routinely in children
presenting with SE [16], especially those with associated fever. Infants
with infection may not have fever and blood counts should be considered
in them, even if afebrile.
Similarly, send blood cultures if the child is
febrile (and above 6 months), or in a younger child, even if afebrile,
if an infection is suspected.
Cerebrospinal fluid (CSF) examination: A central
nervous system (CNS) infection is reported in 12.5% of pediatric
convulsive SE [16]. CNS infections are also an important cause of SE in
Indian children [18]. A CSF examination should be done by lumbar
puncture in a febrile child, after stabilizing the child and excluding
raised intracranial tension [16]. In infants younger than 6 months,
signs of meningitis may not be clearly demonstrated and fever also may
not be present. In such a situation, whenever there is a clinical
suspicion of a CNS infection or sepsis, lumbar puncture should be done.
If done, CSF should be subjected at least to cell
count (total and differential), biochemistry (protein, sugar, CSF: blood
sugar ratio), bacterial culture, and gram stain. CSF pleocytosis, if
present, should not be ascribed to a febrile SE [64]. Additional
investigations on the CSF should be individualized. Systemic illness is
a common trigger for convulsive SE in a patient who is already at risk,
and, therefore, fever itself is not an indication to perform a lumbar
puncture in a patient with epilepsy presenting with SE [58].
Antiepileptic Drug Levels
Inadequate AED drug levels (whether due to
non-compliance, missed dose, or recent drug-dose alterations) are
associated with a significant proportion of SE in children [9], although
some studies found contradictory results [65]. Low AED levels were found
in more than 30% childhood SE, although this was not necessarily the
cause of SE [16].
AED levels should be done, if feasible, in all
patients receiving AED and presenting with SE, as it has both etiologic
(non-compliance/low drug-level as a cause) and therapeutic (loading dose
of the previously effective drug for management) implications. However,
availability of required facilities is likely to act as a bottleneck.
Electroencephalography (EEG)
While considering EEG in SE, two situations need to
be considered viz., an isolated, short-duration single EEG
recording, or continuous EEG monitoring. No Indian studies on usefulness
of EEG in pediatric SE are available. EEG abnormalities have been
reported in ~90% children presenting with SE, though these were done
hours to days later [16]. The information whether the seizure is focal
or generalized is an important one when deciding chronic AED therapy for
the patient.
EEG monitoring has been shown to be extremely useful,
but under-utilized in SE management. After convulsive SE, one-third
of children who undergo EEG monitoring are reported to have
electrographic seizures, and among these, one-third experience entirely
electrographic-only seizures [66,67].
An EEG should be considered in every child presenting
with new-onset SE, although it can be delayed till the control of SE. An
EEG should also be done if there is suspicion of non-convulsive SE
(child not returning to the pre-SE state or remaining persistently
encephalo-pathic even after the control of convulsive SE) or
pseudostatus is suspected. Continuous EEG monitoring optimizes the
management of SE and should be used, if feasible.
Neuroimaging
Neuroimaging can identify structural causes for SE,
especially to exclude the need for neurosurgical intervention in
children with new-onset SE without a prior history of epilepsy, or in
those with persistent SE despite appropriate treatment. MRI is more
sensitive and specific than CT scanning, but CT is more widely available
and quicker in an emergency setting.
A meta-analysis reported structural lesions in 7.8%
of childhood SE, commonly CNS malformations, trauma, and
stroke/hemorrhage [66]. In a more recent study [68], the yield of MRI to
detect structural lesions in convulsive SE was 31%. In the Indian
setting, where inflammatory granulomas are a common cause of seizures
[69], neuroimaging is likely to provide a higher yield.
Neuroimaging should be done, if feasible, in all
children with SE, in whom no definitive etiology has been found. It
should only be done after the child is appropriately stabilized and the
seizure activity controlled. Emergent neuroimaging may be considered if
there are clinical indications (new-onset focal deficits, persistent
altered awareness, fever, recent trauma, history of cancer, history of
anticoagulation, or a suspicion of AIDS).
Special Tests
Metabolic and genetic testing: Inborn errors of
metabolism account for about 4% of SE in children [59]. The common
metabolic causes are listed at Table II. SE usually occurs
during an inter-current illness or metabolic stress [16,70,71].
Pyridoxine dependency can present even after the neonatal period [72],
and is reported in around 0.3% of pediatric SE [16]. This needs to be
excluded by either getting the specific test done (elevated urinary
a-aminoadipic
semialdehyde or the mutations in the ALDH7A1 gene), or giving a
trial of intravenous pyridoxine [72].
TABLE II Metabolic Conditions Associated With Status Epilepticus
|
Group |
Disorders |
|
Mitochondrial diseases |
Myoclonic epilepsy with red ragged fibers (MERRF), Alpers
syndrome, pyruvate dehydrogenase complex deficiency |
|
Lipid storage disorders |
Tay Sachs-Sandhoff disease, Krabbe disease, neonatal
adrenoleukodystrophy, Zellweger syndrome, infantile Refsum
disease, punctuate rhyzomelic chondrodysplasia, Niemann-Pick
disease type A and C, Neuronal ceroid lipofuscinosis |
|
Amino-acidopathies |
Serine metabolism disorders, hyperpolinemia type II, untreated
phenylketonuria, Maple urine syrup diseases, congenital
glutamine deficiency, Nonketotic hyperglycinemia |
|
Organic acidopathies |
Propionic, methylmalonic, D-2- hydroxyglutaric and isovaleric
acidurias; 2-methyl-3-hydroxybutyril-CoA dehydrogenase
deficiency |
|
Other diseases |
Vitamin-dependent epilepsies, creatine metabolism dysfunctions,
Menkes disease, disorders of purine and pyrimidine metabolism |
Metabolic and genetic testing should be considered
when no etiology is revealed in initial evaluation and/or the preceding
history is suggestive of a metabolic disorder. The specific studies to
be obtained should be guided by the history and the clinical
examination.
Toxicology: Toxin or drug-ingestion is a cause of
SE that requires urgent specific treatment. Specific serum toxicology
testing should be considered if initial assessment does not yield the
etiology and/or a suggestive history is elicited.
Work-up for autoimmune encephalitis: Patients of
any age who develop rapidly progressing symptoms presenting or
accompanied by seizures or status epilepticus, usually including
behavioral change and memory deficits, with CSF lymphocytic pleocytosis
and/or oligoclonal bands of unclear etiology, and EEG findings of
encephalopathy and/or epileptic activity, should have serum and CSF
studies for antibodies. In some of these disorders, the MRI is often
normal. The diagnosis is established by demonstrating antibodies in
serum and CSF, though occasionally antibodies are detectable only in the
latter [73].
Investigations to detect SE complications and drug
side-effects: The major complications are altered glucose
metabolism, dyselectrolytemias, and metabolic acidosis [62]. Propylene
glycol toxicity (vehicle in diazepam/lorazepam and barbiturates),
Propofol infusion syndrome, immunosuppression due to barbiturate use,
and liver toxicity due to AEDs [62,63] are the major drug side-effects
seen. These are detailed in Web Table II.
E. Pharmacotherapy
The goal of treatment is the immediate termination of
seizures. For this, drugs should be used in quick succession, and if
possible, rapid institution of pharmacological coma should be done in
refractory cases. In the acute setting, anticonvulsants are best
administered by the intravenous route. Alternative routes can be
employed, to avoid delay in institution of therapy, especially in the
pre-hospital settings. Pharmacotherapy of SE includes drug-management in
the hospital, and management of refractory and super-refractory SE.
Before starting pharmacotherapy for SE in the hospital, the pre-hospital
drugs and doses should be taken in consideration e.g., a child who has
received one dose of midazolam during transfer should receive only one
more dose of midazolam before moving on to the next drug. Table
III provides the guidelines for using various AEDs for SE
management, and Table IV shows the recommended protocol,
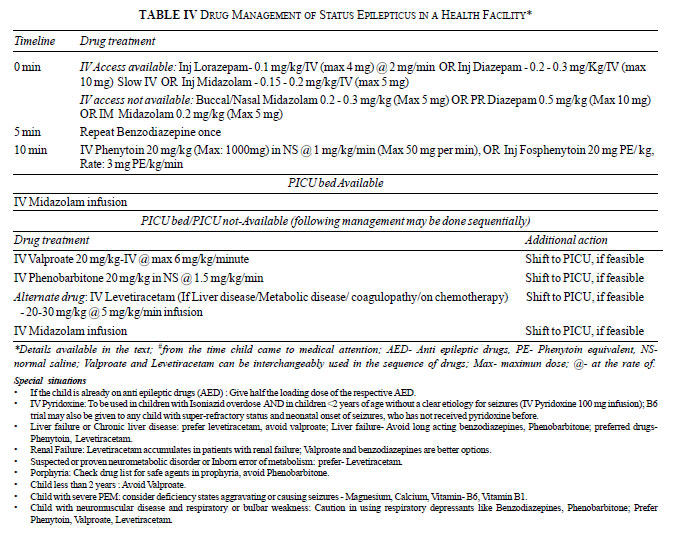
with Box 3 providing supplementary management options.
TABLE III Anticonvulsant Usage in Status Epilepticus
|
Drug
|
Dosage and route |
Comments |
|
Lorazepam |
0.1 mg/Kg/IV (max 4 mg) @ 2 mg/min |
Long acting benzodiazepine, Side effects: sedation, respiratory
depression and hypotension.
|
Midazolam
|
0.15-0.2 mg/Kg;/IV or IM (Max 5 mg)
|
Can be used by IM route.
|
|
Buccal/Nasal: 0.2 - 0.3 mg/Kg (Max 5 mg)
|
|
|
Diazepam |
0.2-0.3 mg/Kg (Max 10 mg) IV |
IV dose should be given slowly over 2-5 min under careful
|
|
0.5 mg/Kg per rectal (Max 10 mg) |
monitoring. |
Phenytoin
|
20 mg/Kg (Max: 1000 mg) in NS @
|
Must be diluted in saline. Side effects include; hypotension,
cardiac |
|
1 mg/Kg/min (Max 50 mg per min)
|
arrythmias, ‘purple glove syndrome’, skin rashes.
Contraindicated in severe hypotension and grade II AV block. |
|
Fosphenytoin |
20 PE/Kg, Rate: 3 PE/Kg/min |
Fewer side effects compared with phenytoin. Can be given IM.
|
|
Valproate |
20 mg/Kg-IV infusion over 15 min,
|
Avoid in presence of liver disease, coagulopathy, |
|
max rate- 6 mg/Kg/min. Followed by
|
thrombocytopenia, suspected metabolic disease, and in infants. |
|
an infusion of 1-2 mg/Kg/h |
|
|
Phenobarbitone |
20 mg/Kg in NS @ 1.5 mg/Kg/min |
Side effects: sedation, respiratory depression, and hypotension |
|
Levetiracetam |
20-30 mg/Kg, over 15 min |
Considered safe in children with metabolic diseases, oncology
patients, and in those with liver disease or coagulopathy.
|
|
Thiopentone |
Induction: 3 mg/Kg bolus, repeated after
|
Causes respiratory depression. Can also induce hypotension and |
|
2 min, followed by maintenance 1-5 mg/Kg/hr |
heart failure, associated with an increased rate of nosocomial |
|
(increasing 1 mg/Kg/hr every 2 min) to
|
infections. Contraindicated in the presence of hypotension, |
|
control seizures and/or to achieve
|
cardiogenic shock and sepsis. |
|
“suppression-burst” EEG activity
|
|
|
Topiramate |
Initial dose: 5-10 mg/Kg/day orally,
|
Side effects: metabolic acidosis, decreased sweating and
glaucoma. |
|
maintenance dose of 5 mg/Kg/day, if effective |
|
|
NS- normal saline, PE: phenytoin equivalents, Max- maximum,
IV –intravenous, IM- intramuscular, @- at the rate. |
BOX 3 Supplementary Management Options in S
|
|
Indications for Mechanical Ventilation |
|
Glasgow coma scale score <8 |
|
Respiratory depression (irregular jerky breathing or apnea) due
to SE or anesthetic agents |
|
Fluid-refractory shock |
|
Raised intracranial pressure
|
|
Difficult-to-maintain airway |
|
Indications for Continuous EEG Monitoring |
|
Prolonged altered sensorium following cessation of clinical
seizures*
|
|
Clinical suspicion of non-convulsive status epilepticus^ |
|
All children receiving IV anesthetic agents# |
|
*No definite time cut-off for “prolonged”; #for
titration of dosage till electroclinical seizure cessation is
achieved; and to monitor for recurrence of electrographic
seizures during tapering; ^Subtle twitching movements of
eyelids, extremities, nystagmoid movements, unexplained
tachycardia in the absence of pulmonary or cardiac pathology. |
Status Epilepticus
A number of anticonvulsants are available and one can
choose the drugs based on availability and cost, and the monitoring
facilities available.
Benzodiazepines: Benzodiazepines are first line
drugs for treatment of SE in children [4,74,75]. The choice within the
benzodiazepines is based on side-effects and pharmacokinetic properties.
Several RCTs and systematic reviews have concluded that lorazepam is the
agent of choice among the benzodiazepines [4,74,75]. More recent data;
however, suggests that the efficacy and side-effect profile of lorazepam
and diazepam is similar in children, when efficacy is defined as
cessation of status epilepticus by 10 minutes without recurrence within
30 minutes [76]. Children receiving lorazepam are less likely to:
require additional doses of anticonvulsants to stop seizures, develop
respiratory depression, and require admission to intensive care unit
[77]. If lorazepam is not available, midazolam or diazepam can be used
for aborting the seizure (Table IV). These two drugs are
shorter acting and thus need to be followed up with longer-acting
non-benzodiazepine anticonvulsants. When using benzodia-zepines, there
is a risk of respiratory depression or arrest, which increases with
repeated doses of the drug [78].
 |
Pheytoin/Fosphenytoin: After using short-acting
benzodiazepines, phenytoin is one of the preferred second-line
anticonvulsant [74]. The loading dose of the drug offers long-duration
seizure-suppression. Major precautions in its use are monitoring for
hypotension and arrhythmias. In addition, local irritation and phlebitis
are common with intravenous administration of phenytoin. Respiratory
depression is exceedingly rare with its use and it does not cause
sedation. In children, care has to be taken to adequately dilute it in
normal saline and as far as possible use a large caliber vessel. As a
second-line AED in SE after benzodiazepines, phenytoin has been
evaluated against phenobarbitone, valproate and levetiracetam
[74,79,80]. However, recent evidence [80] does not support the first
line use of phenytoin.
Fosphenytoin is a water-soluble pro-drug of phenytoin
which has a more favorable side-effect profile and can be given
intramuscularly. It is preferred over phenytoin, when available, but its
higher cost and limited availability precludes its widespread use.
Phenobarbitone
Intravenous phenobarbitone is an effective
alternative to phenytoin in benzodiazepine unresponsive seizures. The
perceived risk of higher rate of respiratory depression after its use
has not been seen in randomized trials [74, 80]. Still, one needs
caution in using it after two or more doses of benzodiazepines. It is
particularly effective in infants younger than one year. When using this
drug, personnel trained in intubation and resuscitation should be
available. Hypotension, respiratory depression and sedation are the
major side-effects. High dose phenobarbitone has also been used for
refractory status epilepticus in intensive care setting [81].
Valproic Acid (Sodium valproate)
The efficacy of valproic acid is similar to phenytoin
after failure of benzodiazepines [82], though a recent meta-analysis
found it to have superior efficacy [80]. In a recent trial in children,
intravenous valproic acid was shown to be equally effective as
phenobarbitone with significant fewer adverse effects [83]. A recent
systematic review of studies with mainly adult patients concluded that
intravenous valproate was as effective as intravenous phenytoin for SE
control [84]. It has also been shown to be effective in children with
status epilepticus refractory to phenytoin [85]. The major advantage of
valproic acid is the relative lack of sedation, respiratory depression
or adverse hemodynamic events. On the other hand, caution needs to be
exercised in its use in infants, and in those with liver disease,
bleeding diathesis and suspected metabolic disorders.
Levetiracetam
This is another emerging drug in the management of
status epilepticus. Presently there are no randomized trials reporting
its use in children. Data in adults suggest that it is as effective as
valproic acid [80,84]. This drug too has the advantage of relative lack
of sedation, respiratory depression or adverse hemodynamic events.
Additionally, it can be used in liver failure and in presence of
bleeding diathesis. It also has the advantage of relatively few
drug-interactions.
An ongoing multi-centric trial is expected to clarify
regarding the best drug (amongst valproate, fosphenytoin and
levetiracetam) to be used after benzodiazepines [86].
Refractory Status Epilepticus
SE is considered refractory if seizures persist
despite the administration of two appropriate anticonvulsants at
acceptable doses [87]. Earlier definitions also mentioned duration of
the status (60 min or 120 min) [88,89]. For the multi-centric Pediatric
Status Epilepticus Research Group (pSERG) study, the definition
described is prolonged seizures that fail to terminate after
administration of two anti-epileptic drugs with different mechanisms of
action or that require continuously administered medication to abort
seizures, regardless of seizure duration [90]. These definitions
highlight the concepts that the potential for neuronal injury is
positively correlated with time, and pharmaco-resistance increases with
time and is reflected in the number of drugs administered [87].
Refractory status epilepticus comprises around 10-40% of patients with
status epilepticus [87,91]. Predictive factors for development of
intractability in patients with SE include encephalitic etiology, severe
impairment of conscious-ness at presentation, absence of a history of
epilepsy, and low anticonvulsant levels (in patients with known
epilepsy) [92,93].
EEG monitoring, if available, is important both to
monitor for electroclinical seizures or non-convulsive electrographic
seizures, and to titrate therapy and the depth of anesthesia, if
necessary [87]. Additional investigations, other than those previously
described, include a high resolution (3 Tesla) MRI to look for cortical
dysplasias, metabolic work-up (blood Tandem mass spectrophotometry
and/or blood/urine Gas chromato-graphy mass spectrophotometry) in young
children, and work-up for autoimmune encephalitis in patients with de
novo status epilepticus associated with fever.
Refractory status epilepticus must be managed in the
intensive care unit. These children require careful cardiorespiratory
monitoring and may also require mechanical ventilation.
Agents available for treatment are anti-epileptic
drugs (non-anesthetic agents) and intravenous anesthetic agents [87].
Non-anesthetic agents include pheno-barbitone, valproic acid,
levetiracetam, topiramate, and lacosamide. Intravenous preparations for
all the above-mentioned drugs, except topiramate are available.
Intravenous anesthetic agents include midazolam, pentobarbital,
thiopental sodium, and propofol. Pentobarbital is not available in
India. Propofol has been used extensively in adult status epilepticus.
However, the risk of propofol infusion syndrome is high in children and
hence propofol is not approved for the treatment of pediatric status
epilepticus in many countries [94]. Given the absence of clear evidence,
the decision to use one or other anesthetic medications must take into
account the patient’s general condition, weighing the benefits against
the potential adverse effects of the medication, and the medical staff‘s
experience in the use of these drugs and their ability to manage the
side effects [95].
Second-line Anticonvulsants: After the failure of
phenytoin/fosphenytoin, trial of any of the following: phenobarbitone,
sodium valproate or levetiracetam may be given. In children below 2
years of age, pyridoxine (100 mg intravenously) may be tried. If the
seizure continues despite this third agent, the patient must be shifted
to the intensive care unit where facilities for mechanical ventilation
and cardiorespiratory monitoring are available. If however, there is a
delay in transfer or intensive care unit is not available, a fourth drug
(phenobarbitone, sodium valproate or levetiracetam; whichever has not
been tried earlier) may be tried before proceeding to midazolam
infusion.
There are reports of use of topiramate in children
with refractory SE leading to rapid resolution of status with no
hemodynamic or sedative side effects. As intravenous preparation is not
available, it should be administered through nasogastric tube [96].
Anecdotal reports of efficacy of Lacosamide in children with SE exist
[97]; however, more data is needed before its use can be recommended.
Intravenous Anesthetic Agents: Midazolam infusion
is the most preferred initial treatment in children with refractory
status epilepticus, effective in seizure control in 76% of these
patients [5]. Midazolam is a short-acting benzodiazepine that rapidly
equilibrates across the blood-brain barrier and has a short elimination
half-life. It has a favorable pharmacokinetic profile which allows for
repeat bolus dosing, aggressive titration of the infusion, and
relatively fast recovery time [98]. It causes little hypotension, and
vasopressors are usually only needed when high doses of midazolam are
used.
Initial effectiveness in terminating pediatric RSE
has been shown in several studies with efficacy rates of approximately
80% to 90% [99,100]. Midazolam should be given as a 0.2 mg /kg bolus
then infusion at the rate of 1 µg/kg/min, increasing 1 µg/kg/min, every
5-10 min, till seizures stop, up to a maximum of 12µg/kg/min [101].
Larger initial bolus doses (0.5 mg/kg) and more aggressive upward dose
titration (up to 2 mg/kg/hour) may result in faster termination of
status epilepticus [87,98,100]. Doses up to 36µg/kg/min have been used
in previous studies, and may be tried provided it is being used in an
ICU setting and appropriate monitoring and management facilities are
available. Tapering should be started 24-48 hours after seizure stops at
the rate of 1 µg/kg/min, every 3-4 hours. Although generally effective
and well tolerated, a drawback of midazolam is the apparent increased
propensity for seizure recurrence on tapering, compared with other
intravenous anesthetic agents.
Thiopental sodium penetrates the central nervous
system rapidly, allowing for rapid titration to EEG burst-suppression.
It has multiple actions: activation of the GABA receptor; inhibition of
N-methyl-D-aspartate (NMDA) receptors; and, alteration in the
conductance of chloride, potassium, and calcium ion channels [5]. Its
prolonged infusion results in a transition from the usual first-order
elimination kinetics seen with bolus doses to the unpredictable
zero-order kinetics and a prolonged elimination half-life because of
distribution in lipid. This phenomenon makes recovery time prolonged and
the drug effect can last days, even with short infusion periods of 12 to
24 hours.
Induction of barbiturate coma is done with bolus of 3
mg/kg, repeated after 2 min, followed by maintenance (1-5 mg/kg/hr) to
control seizures and/or to achieve "suppression-burst" EEG activity
(increasing 1 mg/kg/hr every 2 minutes) [95]. The subsequent maintenance
infusion should continue for 12-48 hours. Thiopental usually causes
respiratory depression. It can also induce hypotension and heart
failure, and inotropic support is frequently needed. Thiopental is
associated with an increased rate of nosocomial infection, especially
pneumonia, and ileus [102]. It is contraindicated in the presence of
hypotension, cardiogenic shock and sepsis [95]. It is reported to
control seizures in 65% of the refractory SE patients not responding to
midazolam [97].
Super-refractory Status Epilepticus
Around 15% of all those presenting to hospital in SE
develop super-refractory status epilepticus and the mortality is 30-50%
[102,103].
Therapies for this entity have not been well studied.
Treatment modalities depend on the availability of resources, and
experience and familiarity of the treating physicians with the various
modalities. Other than the previously mentioned drugs; the agents and
modalities that have been tried in super-refractory status epilepticus
include ketamine [104], inhalational halogenated anesthetics [105],
magnesium infusion [106], steroids and immunotherapy [103], ketogenic
diet [107], hypothermia [108], electrical and magnetic stimulation
therapies [103], electroconvulsive therapy [109], and CSF drainage
[110]. Emergency neurosurgery may be considered in children in whom a
lesion has been detected as the cause of status epilepticus, e.g.
cortical dysplasia [111].
F. Febrile Status Epilepticus
It is defined as status epilepticus in a child aged 1
month to 5 years that also meets the definition of a febrile seizure
[112]. Thus it is clear that febrile central nervous system infections
associated with status epilepticus will not be included in febrile SE.
Febrile SE occur in 5% of febrile seizures [113]. Western studies report
febrile SE as the most common cause of status epilepticus in children
(up to 50%) [114], although Indian studies have reported it to be less
common (10%) [18], or have not characterized it separately [8]. Many of
the issues related to investigations in febrile SE and its outcome are
being explord by the FEBSTAT study [112].
CSF changes in Febrile SE: Pleocytosis due to SE
or febrile SE has long been a controversial issue, and probably the
definitive answer is now available with the results of the FEBSTAT study
[112]. The CSF results from this large group of patients with prolonged
febrile seizure were usually normal: 96% had
Ł5 WBCs/ mm3;
CSF glucose and protein levels were also unremarkable [64]. Human
herpesvirus-6B has been reported to be the most common cause of febrile
SE [115].
Management of febrile SE is similar to that
recommended for SE in these guidelines; however, there is evidence to
show that phenytoin is less efficacious in this situation [116].
G. Management Following Status Epilepticus
It is well-established that the duration of the first
seizure does not affect the risk of recurrence, whether it is a single
seizure or a status epilepticus. Moreover, remission rates are also not
different in those who present with an episode of SE [117]. Brief
recommendations for follow-up management after control of SE are
provided in Box 4.
BOX 4 Guidelines for Follow-up Management of Children with Status Epilepticus
New-onset SE: Further treatment decisions should be similar to
that for a First seizure.
Acute symptomatic seizures: Further treatment depends on the
control of the precipitating event.
SE in known epilepsy:
• After control of SE for 24 hours, tapering of drugs should be
started with ‘last in, first out’ as the guiding principle.
• All the AEDs should preferably be stopped during hospital stay
and the child discharged on:
– Augmented dose of the previous AED/s (if levels were
sub-therapeutic or prescribed dose was less than maximum
dose); and
– Introduction of another appropriate AED (either replacement
or addition), if previously receiving maximum doses of AED/s. |
H. Research Needs
During the deliberations, the group also tried to
identify the areas requiring research in the Indian context. These are
listed in Box 5, and are expected to provide guidance to
the researchers about issues needing evidence-support.
|
BOX 5 Research Needs for Status Epilepticus in
Children |
Epidemiology of SE in India
Role of hypocalcemia in SE, especially in infants
Role of phenobarbitone and phenytoin as the initial AED after
benzodiazepine
Management of SE-associated with neuroinfections
Outcome of SE in Indian children |
Contributors: All members of the writing group
were involved in all aspects of manuscript preparation, approval of the
final manuscript and the decision to publish.
Funding: Association of Child Neurology,
Indian Council of Medical Research, and Sanofi-India Pvt Ltd.
Competing interests: None stated.
Important information: The
participation in the meeting and its deliberations by all the invited
experts was done in their individual capacity, and should not be
considered as the official position of their respective Institutions, or
Professional bodies to which they belong as office-bearers or members.
ANNEXURE I
Participants of the Multi-disciplinary Consensus
Development Workshop on Management of Status Epilepticus in Children in
India
Experts (in alphabetical order): Anju
Aggarwal, UCMS, Delhi; Satinder Aneja, LHMC, Delhi (Convener); B
Chakravarty, AIIMS, Delhi; A Chattopadhyay, Apollo hospital, Kolkata; JS
Goraya, Dayanand Medical College, Ludhiana; Rahul Jain, Chacha Nehru Bal
Chikitsalaya, Delhi; Sourabh Jain, SZ Hospital, Bhopal; Urmila Jhamb,
MAMC, Delhi; Veena Kalra, Delhi; Mahesh Kamate, JNMC, Belgaum; Sujata
Kanhere, KJ Somaiya Medical College, Mumbai; Praveen Khilnani, BLK
Memorial hospital, Delhi; Ramesh Konanki, Hyderabad; Rashmi Kumar, KGMC,
Lucknow; PAM Kunju, Thiruvanantpuram; Lokesh Lingappa, Hyderabad; MM
Mehndiratta, JSSH, Delhi; Rekha Mittal, Max hospital, Delhi; D Mishra,
MAMC, Delhi (Co-convener); V Murugan, Chennai; Rajniti Prasad,
BHU, Varanasi; Ashalatha Radhakrishnan, SCTIMST, Trivandrum; Col. KS
Rana, Military Hospital, Jabalpur; Naveen Sankhyan, PGIMER, Chandigarh;
Suvasini Sharma, LHMC, Delhi; Sunit Singhi, PGIMER, Chandigarh; Sanjib
Sinha, NIMHANS, Bangalore; Bibek Talukdar, CNBC, Delhi; Manjari
Tripathi, AIIMS, Delhi; Vrajesh Udani, Hinduja Hospital, Mumbai; and
Nitish Vora, Ahmedabad.
Rapporteur: Rachna Sehgal, Safdarjung
Hospital, Delhi.
Observers: Puneet Jain, Bijoy Patra, Dinesh
Raj and Harikishan Suthar (all Delhi); Neetu Sharma (Gwalior).
Invited but could not attend the meeting: CP
Bansal, President, Indian Academy of Pediatrics; Virender Kumar, LHMC,
Delhi; Sheffali Gulati, AIIMS, Delhi; Rakesh Lodha, AIIMS, Delhi;
Pratibha Singhi, PGIMER, Chandigarh; Kalpana V, GMC, Thiruvanathapuram;
and Jitendra Sahu, PGIMER, Chandigarh.
|
Key Messages
• Each institution should use a uniform,
written protocol for management of status epilepticus.
• Pre-hospital management and early
stabilization is the key to a satisfactory outcome of status
epilepticus.
• Initial management of status
epilepticus consists of a parenteral benzodiazepine; any
agent by any route may be used depending on the
availability.
• Pharmacotherapy should not be delayed
for any investigations.
• There is a need for more epidemiological research on status
epilepticus from India.
|
References
1. Pellock JM. Status epilepticus in children:
update and review. J Child Neurol. 1994;9:27-35.
2. De Lorenzo RJ. Status epilepticus: concepts in
diagnosis and treatment. Semin Neurol. 1990;10:396-405.
3. Abend NS, Gutierrez-Colina AM, Dlugos DJ.
Medical treatment of pediatric status epilepticus. Semin Pediatr
Neurol. 2010;17:169-75.
4. Appleton R, Macleod S, Martland T. Drug
management for acute tonic-clonic convulsions including convulsive
status epilepticus in children. Cochrane Database Syst Rev.
2008;3:CD001905.
5. Wilkes R, Tasker RC. Pediatric intensive care
treatment of uncontrolled status epilepticus. Crit Care Clin.
2013;29:239-57.
6. Expert Committee on
Pediatric Epilepsy, Indian Academy of Pediatrics. Guidelines for
diagnosis and management of childhood epilepsy. Indian Pediatr.
2009;46:681-98.
7. GEMIND. Guidelines for management of epilepsy
in India. Available from: www.epilepsyindia.com/gemind-book/.
Accessed on 23 July, 2013.
8. Sasidaran K, Singhi S, Singhi P. Management of
acute seizure and status epilepticus in pediatric emergency. Indian
J Pediatr. 2012;79:510-7.
9. Morton LD, Pellock JM. Status Epilepticus.
In: Swaiman KF, Ashwal S, Ferriero DM, Nina F Schor NF.
Swaiman’s Pediatric Neurology: Principles and Practice, 5e; Elsevier
2012.p.798-810.
10. Sharma S, Mishra D, Aneja S, Kumar R, Jain A,
Vashishtha VM, et al. for the Expert Group on Encephalitis,
Indian Academy of Pediatrics. Consensus guidelines on evaluation and
management of suspected acute viral encephalitis in children in
India. Indian Pediatr. 2012;49:897-910.
11. Epilepsy Foundation of America’s Working
Group on Status Epilepticus. Treatment of convulsive status
epilepticus. Recommendations of the Epilepsy Foundation of America’s
Working Group on Status Epilepticus. J Am Med Assoc. 1993;
270:854-59.
12. Commission on Epidemiology and Prognosis,
International League Against Epilepsy. Guidelines for epidemiologic
studies on epilepsy. Epilepsia. 1993;34:592-96.
13. Lowenstein DH, Bleck T, Macdonald RL. It’s
time to revise the definition of status epilepticus. Epilepsia.
1999; 40:120-2.
14. Shinnar S, Berg AT, Moshe SL, Shinnar R. How
long do new-onset seizures in children last? Ann Neurol. 2001;
49:659-64.
15. Capovilla G, Beccaria F, Beghi E, Minicucci
F, Sartori S, Vecchi M. Treatment of convulsive status epilepticus
in childhood: Recommendations of the Italian League Against
Epilepsy. Epilepsia. 2013;54:23-34.
16. Riviello JJ, Ashwal S, Hirtz D, Glauser T,
Ballaban-Gil K, Kelley K, et al. Practice Parameter:
Diagnostic Assessment of the Child With Status Epilepticus (an
evidence-based review). Report of the Quality Standards Subcommittee
of the American Academy of Neurology and the Practice Committee of
the Child Neurology Society. Neurology. 2006;67:1542-50.
17. Chin RF, Neville BG, Peckham C, Bedford H,
Wade A, Scott RC. Incidence, cause, and short-term outcome of
convulsive status epilepticus in childhood: prospective
population-based study. Lancet. 2006;368:222–29.
18. Gulati S, Kalra V, Sridhar MR.
Status epilepticus in Indian children in a tertiary care center.
Indian J Pediatr. 2005;72:105-8.
19. Tripathi M, Vibha D, Choudhary N, Prasad K,
Srivastava MV, Bhatia R, et al. Management of refractory
status epilepticus at a tertiary care centre in a developing
country. Seizure. 2010;19:109-11.
20. Sinha S, Satishchandra P, Mahadevan A,
Bhimani BC, Kovur JM, Shankar SK. Fatal status epilepticus: A
clinico-pathological analysis among 100 patients: from a developing
country perspective. Epilepsy Res. 2010;91:193-204.
21. Pellock JM. Overview: definitions and
classifications of seizure emergencies. J Child
Neurol. 2007;22:9S-13S.
22. Chin RF, Verhulst L, Neville BG, Peters
MJ, Scott RC. Inappropriate emergency management of status
epilepticus in children contributes to need for intensive care. J
Neurol Neurosurg Psychiatry. 2004;75:1584-8.
23. Facility Based IMNCI (F-IMNCI)
Participants Manual. Government of India. Available from:
www.unicef.org/india/FBC_Participants_Manual. Accessed August
31, 2014.
24. Alldredge BK, Gelb AM, Isaacs SM, Corry MD,
Allen F, Ulrich S, et al. A comparison of lorazepam,
diazepam, and placebo for the treatment of out-of-hospital status
epilepticus. N Engl J Med. 2001;345:631-7.
25. Ahmad S, Ellis JC, Kamwendo H, Molyneux E.
Efficacy and safety of intranasal lorazepam versus intramuscular
paraldehyde for protracted convulsions in children: an open
randomized trial. Lancet. 2006;367:1591-7.
26. Dreifuss FE, Rosman NP, Cloyd JC, Pellock JM,
Kuzniecky RI, Lo WD, et al. A comparison of rectal diazepam
gel and placebo for acute repetitive seizures. N Engl J Med.
1998;338:1869-75.
27. Cereghino JJ, Mitchell WG, Murphy J, Kriel
RL, Rosenfeld WE, Trevathan E. Treating repetitive seizures with a
rectal diazepam formulation: a randomized study. The North American
Diastat Study Group. Neurology. 1998;51:1274-82.
28. Pellock JM. Safety of Diastat, a rectal gel
formulation of diazepam for acute seizure treatment. Drug Saf. 2004;
27:383-92.
29. Holsti M, Sill BL, Firth SD, Filloux FM,
Joyce SM, Furnival RA. Prehospital intranasal midazolam for the
treatment of pediatric seizures. Pediatr Emerg Care. 2007;23:148-53.
30. Kutlu NO, Dogrul M, Yakinci C, Soylu H.
Buccal midazolam for treatment of prolonged seizures in children.
Brain Dev. 2003;25:275-8.
31. Scott RC, Besag FM, Boyd SG, Berry D, Neville
BG. Buccal absorption of midazolam: pharmacokinetics and EEG
pharmacodynamics. Epilepsia. 1998;39:290-4.
32. Ashrafi MR, Khosroshahi N, Karimi P, Malamiri
RA, Bavarian B, Zarch AV, et al. Efficacy and usability
of buccal midazolam in controlling acute prolonged convulsive
seizures in children. Eur J Paediatr Neurol. 2010;14:434-8.
33. Wiznitzer M. Buccal midazolam for seizures.
Lancet. 2005;366:182-3.
34. Kendall JL, Reynolds M, Goldberg R.
Intranasal midazolam in patients with status epilepticus. Ann Emerg
Med. 1997;29:415-7.
35. Jeannet PY, RouletE, Maeder-Ingvar M, Gehri
M, Jutzi A, Deonna T. Home and hospital treatment of acute seizures
in children with nasal midazolam. Eur J Paediatr Neurol.
1999;3:73-7.
36. Silbergleit R, Durkalski V, Lowenstein D,
Conwit R, Pancioli A, Palesch Y, Barsan W; NETT Investigators.
Intramuscular versus intravenous therapy for prehospital status
epilepticus. N Engl J Med. 2012;366:591-600.
37. Arya R, Gulati S, Kabra M, Sahu JK, Kalra V.
Intranasal versus intravenous lorazepam for control of acute
seizures in children: a randomized open-label study. Epilepsia.
2011;52:788-93.
38. Ma L, Yung A, Yau Ee, Kwong K. Clinical
Guidelines on Management of Prolonged Seizures, Serial Seizures and
Convulsive Status Epilepticus in Children. HK J Paediatr (New
Series) 2010;15:52-63.
39. Shorvon S, Ferlisi M. The treatment of
super-refractory status epilepticus: a critical review of available
therapies and a clinical treatment protocol. Brain J Neurol.
2011;134:2802-18.
40. Capovilla G, Beccaria F, Beghi E, Minicucci
F, Sartori S, Vecchi M. Treatment of convulsive status epilepticus
in childhood: Recommendations of the Italian League Against
Epilepsy. Epilepsia. 2013; 54:23-34.
41. Wijdicks E. Neurologic catastrophies in the
emergency department. Boston: Butterworth-Heinemann; 2000.
42. Lothman E. The biochemical basis and
pathophysiology of status epilepticus. Neurology. 1990;40:13-23.
43. Shorvon SD. Emergency treatment of acute
seizures, serial seizures, seizure cluster and status epilepticus.
In: Shorvon SD. Handbook of Epilepsy Treatment. Oxford:
Blackwell Science; 2000.
44. Fountain NB. Status epilepticus: risk factors
and complications. Epilepsia. 2000;41:S23-30.
45. Fountain NB, Lothman EW. Pathophysiology of
status epilepticus. J Clin Neurophysiol. 1995;12:326-42.
46. Simon RP. Physiologic consequences of status
epilepticus. Epilepsia. 1985;26:S58-66.
47. Wijdicks EFM. The multifaceted care of status
epilepticus. Epilepsia. 2013;54:61-3.
48. Sutter R, Tschudin-Sutter S, Grize L, Fuhr P,
Bonten MJ, Widmer AF, et al. Associations between infections
and clinical outcome parameters in status epilepticus: a
retrospective 5-year cohort study. Epilepsia. 2012;53:1489-97.
49. Bleck TP. Intensive care unit management of
patients with status epilepticus. Epilepsia. 2007;48:59-60.
50. Vespa PM, Miller C, McArthur D, Eliseo
M, Etchepare M, Hirt D, et al. Nonconvulsive electrographic
seizures after traumatic brain injury result in a delayed, prolonged
increase in intracranial pressure and metabolic crisis. Crit Care
Med. 2007;35:2830-6.
51. Weeks SG, Alvarez N, Pillay N, Bell RB.
Takotsubo cardiomyopathy secondary to seizures. Can J Neurol Sci.
2007;34:105-7.
52. Stöllberger C, Wegner C, Finsterer J.
Seizure-associated Takotsubo cardiomyopathy. Epilepsia.
2011;52:e160-7.
53. Lemke DM, Hussain SI, Wolfe TJ, Torbey
MA, Lynch JR, Carlin A, et al. Takotsubo cardiomyopathy
associated with seizures. Neurocrit Care. 2008;9:112-7.
54. Boggs JG, Painter JA, DeLorenzo RJ. Analysis
of electrocardiographic changes in status epilepticus. Epilepsy Res.
1993;14:87-94.
55. Manno EM, Pfeifer EA, Cascino GD, Noe KH,
Wijdicks EFM. Cardiac pathology in status epilepticus. Ann Neurol.
2005;58:954-7.
56. Luck RP, Verbin S. Rhabdomyolysis: a review
of clinical presentation, etiology, diagnosis, and management.
Pediatr Emerg Care. 2008;24:262-8.
57. Gunn V, Nechyba C. The Harriet Lane Handbook.
16th Ed. St Louis, MO: Mosby Elseiver, Inc; 2002.
58. Freilich ER, Zelleke T, Gaillard WD.
Identification and evaluation of the child in status epilepticus.
Semin Pediatr Neurol. 2010;17:144-9.
59. Mastrangelo M, Celato A. Diagnostic work-up
and therapeutic options in management of pediatric status
epilepticus. World J Pediatr. 2012;8:109-15.
60. Mehrotra P, Marwaha RK, Aneja S, Seth A,
Singla BM, Ashraf G, et al. Hypovitaminosis D and
hypocalcemic seizures in infancy. Indian Pediatr. 2010;47:581-6.
61. Balasubramanian S, Shivbalan S, Kumar PS.
Hypocalcemia due to vitamin D deficiency in exclusively breastfed
infants. Indian Pediatr. 2006;43:247-51.
62. Shorvon S, Ferlisi M. The treatment of
super-refractory status epilepticus: a critical review of available
therapies and a clinical treatment protocol. Brain.
2011:134;2802-18.
63. Wijdicks EFM. The multifaceted care of status
epilepticus. Epilepsia. 2013;54:61-3.
64. Frank LM, Shinnar S, Hesdorffer DC, Shinnar
RC, Pellock JM, Gallentine W, et al.; FEBSTAT Study Team.
Cerebrospinal fluid findings in children with fever-associated
status epilepticus: results of the consequences of prolonged febrile
seizures (FEBSTAT) study. J Pediatr. 2012;161:1169-71.
65. Maytal J, Novak G, Ascher C, Bienkowski R.
Status epilepticus in children with epilepsy: the role of
antiepileptic drug levels in prevention.
Pediatrics. 1996;98:1119-21.
66. Greiner HM, Holland K, Leach JL, Horn
PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the
encephalopathic pediatric patient. Pediatrics. 2012; 129:e748-55.
67. Sánchez Fernández I, Abend NS, Arndt
DH, Carpenter JL, Chapman KE, Cornett KM, et al.
Electrographic seizures after convulsive status
epilepticus in children and young adults: a retrospective
multicenter study. J Pediatr. 2014;164:339-46.
68. Yoong M, Madari R, Martinoss R, Clark C,
Chong K, Neville B, et al. The role of magnetic resonance
imaging in the follow-up of children with convulsive status
epilepticus. Dev Med Child Neurol. 2012;54:328-33.
69. Singhi P. Neurocysticercosis. Ther Adv Neurol
Disord. 2011;4:67-81.
70. Eriksson KJ, Koivikko MJ. Status epilepticus
in children: Aetiology, treatment, and outcome. Dev Med Child
Neurol. 1997;39:652-8.
71. Hussain N, Appleton R, Thorburn K. Aetiology,
course
and outcome of children admitted to paediatric
intensive care with convulsive status epilepticus: a retrospective
5-year review. Seizure. 2007;16:305-12.
72. Yeghiazaryan NS1, Zara F, Capovilla G,
Brigati G, Falsaperla R, Striano P. Pyridoxine-dependent epilepsy:
An under-recognised cause of intractable seizures. J Paediatr Child
Health. 2012;48:E113-5.
73. Davis R, Dalmau J. Autoimmunity, seizures,
and status epilepticus. Epilepsia 2013;54:46-9.
74. Treiman DM, Meyers PD, Walton NY, Collins
JF, Colling C, Rowan AJ, et al. A comparison of four
treatments for generalized convulsive status epilepticus. Veterans
Affairs Status Epilepticus Cooperative Study Group. N Engl J Med.
1998;339:792-8.
75. Sreenath TG, Gupta P, Sharma KK,
Krishnamurthy S. Lorazepam versus diazepam-phenytoin combination in
the treatment of convulsive status epilepticus in children: A
randomized controlled trial. Eur J Paediatr Neurol. 2010;14:162-8.
76. Chamberlain JM, Okada P, Holsti M, Mahajan
P, Brown KM, Vance C, et al., Pediatric Emergency Care
Applied Research Network (PECARN). Lorazepam vs diazepam for
pediatric status epilepticus: A randomized clinical trial.
JAMA. 2014;311:1652-60.
77. Appleton R, Sweeney A, Choonara I, Robson J,
Molyneux E. Lorazepam versus diazepam in the acute treatment of
epileptic seizures and status epilepticus. Dev Med Child Neurol.
1995;37:682-8.
78. Stewart WA, Harrison R, Dooley JM.
Respiratory depression in the acute management of seizures. Arch Dis
Child. 2002;87:225-6.
79. Agarwal P, Kumar N, Chandra R, Gupta G,
Antony AR, Garg N. Randomized study of intravenous valproate and
phenytoin in status epilepticus. Seizure. 2007;16:527-32.
80. Yasiry Z, Shorvon SD. The relative
effectiveness of five antiepileptic drugs in treatment of
benzodiazepine-resistant convulsive status epilepticus: A
meta-analysis of published studies. Seizure. 2014;23:167-74.
81. Crawford TO, Mitchell WG, Fishman LS,
Snodgrass SR. Very-high-dose phenobarbital for refractory status
epilepticus in children. Neurology. 1988;38:1035-40.
82. Misra UK, Kalita J, Patel R. Sodium valproate
vs phenytoin in status epilepticus: a pilot study. Neurology.
2006;67:340-2.
83. Malamiri RA, Ghaempanah M, Khosroshahi N,
Nikkhah A, Bavarian B, Ashrafi MR. Efficacy and safety of
intravenous sodium valproate versus phenobarbital in controlling
convulsive status epilepticus and acute prolonged convulsive
seizures in children: A randomised trial. Eur J Paediatr Neurol.
2012;16:536-41.
84. Liu X, Wu Y, Chen Z, Ma M, Su L. A systematic
review of randomized controlled trials on the theraputic effect of
intravenous sodium valproate in status epilepticus. Int J Neurosci.
2012;122:277-83.
85. Mehta V, Singhi P, Singhi S. Intravenous
sodium valproate versus diazepam infusion for the control of
refractory status epilepticus in children: A randomized controlled
trial. J Child Neurol. 2007;22:1191-7.
86. Cock HR; ESETT Group. Established status
epilepticus treatment trial (ESETT). Epilepsia. 2011;52:50-2.
87. Owens J. Medical management of refractory
status epilepticus. Semin Pediatr Neurol. 2010;17:176-81.
88. Mayer SA, Claassen J, Lokin J, Mendelsohn F,
Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: Frequency,
risk factors, and impact on outcome. Arch Neurol. 2002;59:205-10.
89. Stecker MM, Kramer TH, Raps EC, O’Meeghan R,
Dulaney E, Skaar DJ. Treatment of refractory status epilepticus with
propofol: Clinical and pharmacokinetic findings. Epilepsia.
1998;39:18-26.
90. Sánchez Fernández I, Abend NS, Agadi S, An S,
Arya R, Carpenter JL, et al. Gaps and opportunities in
refractory status epilepticus research in children: A multi-center
approach by the Pediatric Status Epilepticus Research Group [pSERG].
Seizure. 2014;23:87-97.
91. Abend NS, Dlugos DJ. Treatment of refractory
status epilepticus: Literature review and a proposed protocol.
Pediatr Neurol. 2008;38:377-90.
92. Drislane FW, Blum AS, Lopez MR, Gautam
S, Schomer DL. Duration of refractory status epilepticus and
outcome: Loss of prognostic utility after several hours. Epilepsia.
2009;50:1566-71.
93. Holtkamp M, Othman J, Buchheim K, Meierkord
H. Predictors and prognosis of refractory status epilepticus treated
in a neurological intensive care unit. J Neurol Neurosurg
Psychiatry. 2005; 76:534-9.
94. Wilkes R, Tasker RC. Pediatric intensive care
treatment of uncontrolled status epilepticus. Crit Care Clin.
2013;29:239-57.
95. Capovilla G, Beccaria F, Beghi E, Minicucci
F, Sartori S, Vecchi M. Treatment of convulsive status epilepticus
in childhood: recommendations of the Italian League Against
Epilepsy. Epilepsia. 2013;54:23-34.
96. Shiloh-Malawsky Y, Fan Z, Greenwood R,
Tennison M. Successful treatment of childhood prolonged refractory
status epilepticus with lacosamide. Seizure. 2011;20: 586-8.
97. Wilkes R, Tasker RC. Intensive care treatment
of uncontrolled status epilepticus in children: Systematic
literature search of midazolam and anesthetic therapies. Pediatr
Crit Care Med. 2014 Jun 3. [Epub ahead of print]
98. Koul R, Chacko A, Javed H, Al Riyami K.
Eight-year study of childhood status epilepticus: Midazolam infusion
in management and outcome. J Child Neurol. 2000;17:908-10.
99. Morrison G, Gibbons E, Whitehouse WP.
High-dose midazolam therapy for refractory status epilepticus in
children. Intensive Care Med. 2006;32:2070-6.
100. Raj D, Gulati S, Lodha R. Status
epilepticus. Indian J Pediatr. 2011;78:219-26.
101. Holmes GL, Riviello JJ Jr. Midazolam and
pentobarbital for refractory status epilepticus. Pediatr Neurol.
1999;20:259-64.
102. Shorvon S, Ferlisi M. The treatment of
super-refractory status epilepticus: a critical review of available
therapies and a clinical treatment protocol. Brain. 2011;134:
2802-18.
103. Ferlisi M, Shorvon S. The outcome of
therapies in refractory and super-refractory convulsive status
epilepticus and recommendations for therapy. Brain.
2012;135:2314-28.
104. Rosati A, L’Erario M, Ilvento L, Cecchi C,
Pisano T, Mirabile L, Guerrini R. Efficacy and safety of ketamine in
refractory status epilepticus in children. Neurology.
2012;79:2355-8.
105. Mirsattari SM, Sharpe MD, Young GB.
Treatment of refractory status epilepticus with inhalational
anesthetic agents isoflurane and desflurane. Arch Neurol.
2004;61:1254-9.
106. Visser NA, Braun KP, Leijten FS, van
Nieuwenhuizen O, Wokke JH, van den Bergh WM. Magnesium treatment for
patients with refractory status epilepticus due to POLG1-mutations.
J Neurol. 2011;258:218-22.
107. Cobo NH, Sankar R, Murata KK, Sewak SL,
Kezele MA, Matsumoto JH. The ketogenic diet as broad-spectrum
treatment for super-refractory pediatric status epilepticus:
challenges in implementation in the Pediatric and Neonatal Intensive
Care Units. J Child Neurol. 2014 Jan 23. [Epub ahead of print]
108. Guilliams K, Rosen M, Buttram S, Zempel J,
Pineda J, Miller B, Shoykhet M. Hypothermia for pediatric refractory
status epilepticus. Epilepsia. 2013;54: 1586-94.
109. Lambrecq V, Villéga F, Marchal C, Michel V,
Guehl D, Rotge JY, Burbaud P. Refractory status epilepticus:
electroconvulsive therapy as a possible therapeutic strategy.
Seizure. 2012;21:661-4.
110. Köhrmann M, Huttner HB, Gotthardt D, Nagel
S, Berger C, Schwab S. CSF-air-exchange for pharmacorefractory
status epilepticus. J Neurol. 2006;253:1100-1.
111. Vendrame M, Loddenkemper T. Surgical
treatment of refractory status epilepticus in children: candidate
selection and outcome. Semin Pediatr Neurol. 2010;17:182-9.
112. Hesdorffer DC, Shinnar S, Lewis DV, Nordli
DR, Pellock JM, Moshe SL, Shinnar RC, et al. for the FEBSTAT
Study Team. Risk factors for febrile status epilepticus: A
case-control study. J Pediatr. 2013;163:1147-51.
113. Berg AT, Shinnar S. Complex febrile
seizures. Epilepsia. 1996; 37:126-33.
114. Nishiyama I, Ohtsuka Y, Tsuda T, Kobayashi
K, Inoue H, Narahara K, et al. An epidemiological study of
children with status epilepticus in Okayama, Japan: incidence,
etiologies, and outcomes. Epilepsy Res. 2011;96:89-95.
115. Theodore WH, Epstein L, Gaillard WD, Shinnar
S, Wainwright MS, Jacobson S. Human herpes virus 6B: a possible role
in epilepsy? Epilepsia. 2008;49:1828-37.
116. Ismail S, Lévy A, Tikkanen H, Sévčre M,
Wolters FJ, Carmant L. Lack of efficacy of phenytoin in children
presenting with febrile status epilepticus. Am J Emerg Med. 2012;
30:2000-4.
117. Shinnar S, Berg AT, Moshe SL, O’Dell C, Alemany
M, Newstein D, et al. The risk of seizure recurrence after a
first unprovoked afebrile seizure in childhood: an extended follow-up.
Pediatrics. 1996;98:216-25.
|
|
|
 |
|

