|
|
|
Indian Pediatr 2014;51:
969-974 |
 |
Safety and Efficacy of Isotonic (0.9%) vs.
Hypotonic (0.18%) Saline as Maintenance Intravenous Fluids in
Children: A Randomized Controlled Trial
|
|
Ahmar Shamim, Kamran Afzal and S Manazir Ali
From Department of Pediatrics, Jawaharlal Nehru
Medical College, Aligarh Muslim University, Aligarh,
Uttar Pradesh,
India.
Correspondence to: Dr Ahmar Shamim, Assistant
Professor, Mahatma Gandhi Mission Medical College, Navi Mumbai,
Maharashtra, India.
Email: [email protected]
Received: July 18, 2014;
Initial review: September 26, 2014;
Accepted: October 09, 2014.
Trial registration: CTRI/2010/091/000398.
|
Objective: To compare the safety and efficacy of isotonic versus
hypotonic maintenance fluid in children.
Design: Randomized controlled
trial.
Setting: Tertiary-level teaching
hospital.
Participants: 60 children
(age 0.5 to 12 years) who were admitted and anticipated to receive
intravenous fluid for the next 48 hours.
Intervention: Hypotonic fluid
(Standard maintenance volume as 0.18% NaCl in 5% dextrose) or Isotonic
fluid (60% Standard maintenance volume as 0.9% NaCl solution in 5%
dextrose).
Outcome measures: Primary:
Incidence of hyponatremia. Secondary: Serum sodium, serum
osmolality, blood sugar, blood urea, serum creatinine, serum potassium,
serum chloride, pH, urine output, change in weight, morbidity and death.
Results: At 24 hours, hyponatremia was noted in 7 (24%) patients
in the isotonic and 16 (55%) in hypotonic group (P=0.031). At 48
hours, hyponatremia was noted in 4 (14%) and 13 (45%) patients in
isotonic and hypotonic group, respectively (P=0.02). There was
significant change in sodium levels in both isotonic (P=0.036)
and hypotonic (P<0.001) intervention groups. The peak fall in
mean serum sodium level was noted at 24 hours (-6.5, 95%CI: -3.5, -9.6
mEq/L; P<0.001) in hypotonic group. In isotonic group, there was
significant increase between 24 and 48 hours (4.3, 95% CI: 0.1, 8.4 mEq/L;
P=0.04).
Conclusions: Reduced volume
isotonic fluid results in fewer episodes of hyponatremia than hypotonic
fluid in sick children during the first 48 hours of intravenous fluid
therapy.
Keywords: Hyponatremia,
Intravenous fluids, Normal saline, Parenteral fluid therapy.
|
|
Recommendation for the use of a hypotonic saline
solution (0.18% saline in 5% dextrose) in children is still a debated
subject despite half a century of its practice [1]. Reports of
symptomatic hyponatremia in hospitalized surgical and non-surgical
pediatric patients – caused primarily by various non-osmotic release of
vasopressin, but contributed by electrolyte-free water input in a
proportion of cases – have fueled these debates [2-5]. Use of
conventional volume maintenance isotonic saline has been shown to reduce
the incidence of hyponatraemia [6]. Using indirect calorimetric
measurements, energy expenditure in critically ill children may be as
low as 50-60 kcal/kg/day [7]. Consequently, fluid requirement, which is
directly proportional to the actual energy expenditure, is much less
than previously assumed in critically ill children for a variety of
reasons such as physical immobility, the use of muscle relaxants and
sedatives, mechanical ventilation, and additional factors such as
nonessential or facultative metabolism [8]. Therefore, we hypothesized
that use of reduced volume isotonic maintenance fluid would decrease the
incidence of hyponatremia in sick children, when compared to hypotonic
fluid. We compared the efficacy and safety of isotonic fluid (0.9% NaCl
in 5% dextrose) at the rate of 60% of daily fluid requirement versus
hypotonic fluid (0.18% NaCl in 5% dextrose) at the rate of standard
maintenance volume in sick children.
Methods
This open-label randomized controlled trial was
conducted in the Pediatric ward of Jawahar Lal Nehru Medical College
Hospital in Aligarh, India from November 2009 to October 2010. Children
in the age group of 0.5 to 12 years, who were admitted and anticipated
to receive intravenous fluid for the next 48 hours, were considered for
recruitment into the study. Written informed consent was obtained from
the parent or guardian of all patients before enrolment. The study
protocol was approved by the Institute Ethics Committee.
Children with hyponatremia (serum sodium <130 mEq/L),
hypernatremia (serum sodium >150 mEq/L), acute gastroenteritis,
hemodynamic instability (shock, myocarditisbcongestive heart failure),
acute or chronic kidney disease, a history/evidence of cardiac dysfunction,
uncontrolled seizures, severe developmental delay, diabetes mellitus or insipidus, and severe malnutrition, were excluded. Children with
pre-existing hypertension, diuretic use, edema, or known adrenal
dysfunction, or those who had received intravenous fluid within
preceding 3 hours were also excluded.
All eligible patients were randomized to receive
either hypotonic saline solution (0.18% NaCl in 5% dextrose), at the
rate of standard maintenance volume or isotonic saline solution (0.9%
NaCl solution in 5% dextrose), at the rate of 60% of standard
maintenance volume. Maintenance fluid volume for administration was
calculated using Holliday and Segar formula [1]. Both groups also
received 1 mL of potassium chloride per 100 mL of intravenous fluids.
Randomization sequence was generated (block randomization, size 4) and
maintained by a colleague not directly involved in this trial using an
online computer program. The allocated intervention was kept in
sequentially labeled sealed opaque envelopes to be opened at the time of
randomization.
Baseline demographic, anthropometric and laboratory
characteristics were noted at enrolment. All patients were monitored
clinically for symptoms and signs of dysnatremia, and signs of fluid
overload or dehydration throughout the study period. Clinical
assessment, including weight and urine output was done every 12 hours.
Laboratory measurements included 12-hourly serum sodium, serum
potassium, serum chloride, pH (Combiline Eschweiler blood gas and
electrolyte analyzer), blood urea, serum creatinine and blood sugar.
Serum osmolality (by freeze point depression using Osmomat-30, Gonotech,
Germany) was done at 0, 24 and 48 hours. Participants who developed
symptomatic dysnatremia (serum sodium <130 or >150mEq/L) or asymptomatic
dysnatremia (serum sodium <125 or >155mEq/L), dehydration/features of
hypervolemia, require fluid boluses for volume resuscitation, weight
loss of >5%, increase in blood urea/serum creatinine >10% from baseline
or urine output <1mL/kg/hr at any stage were excluded from the study.
The primary outcome of the study was the incidence of
hyponatremia (defined as serum sodium <130 mEq/L). Incidence of
hypernatremia, serum sodium, serum osmolality, blood sugar, blood urea,
serum creatinine, serum potassium, serum chloride, pH, urine output,
change in weight, morbidity (seizures, cerebral edema or other
neurological manifestations attributable to dys-natremias) and death
were secondary outcome measures.
As the previously reported incidence of hyponatremia
is quite variable, we calculated the sample size from our pilot data of
10 patients per intervention group; hyponatremia was observed in 5
patients on hypotonic fluid (HF) and 1 in isotonic fluid (IF). A sample
size of 25 patients per group was calculated to be adequate to reject
the null hypothesis with a power of 90% and an alpha error of 0.05. To
account for a 15% loss to follow-up, we decided to enroll 30 patients
per intervention group.
The data were analyzed with SPSS version 17.0
software. Analysis was done on an intention-to-treat basis. Ordinal data
was compared using Fisher’s exact test or Chi-square test. Independent
sample t-test was used to compare difference in serum sodium, serum
osmolality, urinary outputs, percentage change in weight, and other
secondary variables, of the two groups in the study. Significance was
taken at P value of <0.05.
Results
During the study period, a total of 480 consecutive
patients were assessed of which 202 met the eligibility criteria. Of
these, 142 children had to be excluded (Fig. 1). Thus a
total of 60 children were randomized, 30 to each treatment intervention
group. Mean (SD) age of the study population was 53.8 (35.5) months; 33
(55%) were male. Baseline characteristics did not differ significantly
between the two intervention groups (Table I). Of the 15
cases with infections, diagnosis was enteric fever (n=4),
complicated malaria (n=8) or sepsis (n=3). The study was
discontinued within 24 hours in 2 patients (one from each group); one
patient in IF developed acute gastroenteritis with dehydration requiring
intravenous rehydration and the other patient in HF developed clinical
features of fluid overload requiring diuretics.
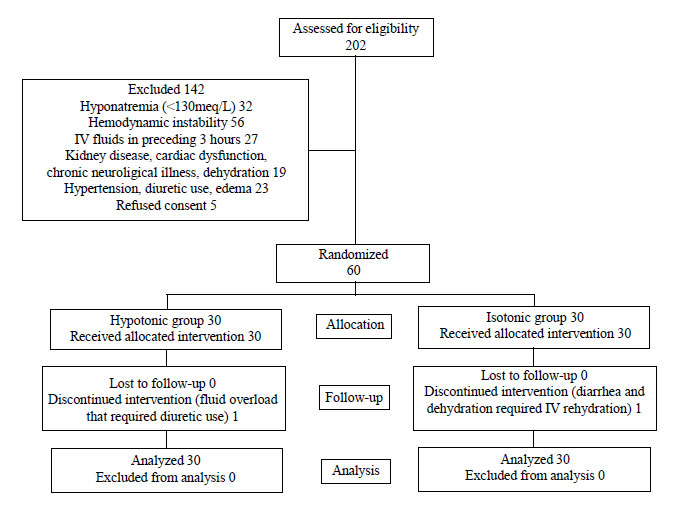 |
|
Fig. 1 Flow of patients in the study.
|
TABLE I Baseline Clinical and Biochemical Characteristics of Participants
Parameter
|
Isotonic
group
(N=30) |
Hypotonic
group
(N=30) |
| |
|
|
|
Age (mo) |
53.1 (39.5) |
54.4 (31.7) |
|
Sex (M:F) |
17:13 |
16:14 |
|
Weight-for-age, % |
79.5 (9.0) |
77.7 (6.7) |
|
Body mass index, Kg/m2 |
14.6 (1.2) |
13.9 (1.0) |
|
Body surface area, m2 |
0.6 (0.20) |
0.6 (0.16) |
|
Serum sodium, mEq/L |
135.7 (4.2) |
136.3 (3.5) |
|
Serum osmolality, mOSm/L |
291.4 (10.0) |
293.8 (8.5) |
|
Blood sugar, mg/dL |
104.3 (13.9) |
99.3 (12.7) |
|
Blood urea, mg/dL |
39.6 (8.6) |
44.2 (9.5) |
|
Serum creatinine, mg/dL |
0.70 (0.08) |
0.77 (0.09) |
|
Serum potassium, mEq/L |
3.9 (0.8) |
4.1 (0.6) |
|
Serum chloride, mEq/L |
99.8 (6.3) |
101.3 (6.6) |
|
pH |
7.36 (0.06) |
7.36 (0.06) |
|
Diagnosis at admission |
|
Meningo-encephalitis |
9 |
11 |
|
Acute respiratory illness |
12 |
11 |
|
Infections |
8 |
7 |
|
Acute hepatitis |
1 |
1 |
|
All values in mean (SD); P>0.05 for all parameters |
|
|
Hyponatremia: The incidence of hyponatremia was
33.3% (n=10) in the IF group and 70% (n=21) in the HF group (RR 0.48,
(95%CI, 0.27, 0.83; P=0.01). The number of children with
hyponatremia at 12, 24, 36 and 48 hours were 3, 7, 3 and 4 in IF and 4,
16, 14 and 13 in HF. The incidence was significantly different at 24 (P=0.031),
36 (P=0.003) and 48 hours (P=0.022).
Of the patients who developed hyponatremia, 9 had
values below 125 mEq/L (2 in IF and 7 in HF). The numbers that had serum
sodium level <125 mEq/L were 3 (2 in HF) at 24 hours and 5 (4 in HF) at
48 hours. There was no patient with serum sodium level below 120mEq/L.
Using serum sodium levels below 135 mEq/L to define hyponatremia, 25 in
HF and 16 in IF were hyponatremic at 24 hours (P=0.009) whereas
at 48 hours, 20 in HF and 6 in IF were hyponatremic (P<0.001).
Hypernatremia was noted at 48 hours in 3 IF (P = 0.27).
TABLE II Laboratory Measurements In Study Participants*
|
Laboratory investigations¶ |
Time (hours) |
IF |
HF |
P value# |
|
Serum sodium (mEq/l) |
0 |
135.7 (134.1,137.4) |
136.3 (135.0,137.7) |
0.62 |
|
24 |
134.6 (132.5,136.8) |
129.7 (128.3,131.1) |
<0.001 |
|
48 |
138.9 (136.0,141.8) |
131.4 (129.2,133.5) |
<0.001 |
|
Serum osmolality(mOsm/l) |
0 |
291.4 (287.7,295.4) |
293.8 (290.8,297.3) |
0.32 |
|
24 |
290.9 (286.1,295.7) |
279.8 (276.6,283.0) |
<0.001 |
|
48 |
300.7 (294.8,306.6) |
282.2 (277.8,286.6) |
<0.001 |
|
Blood urea(mg/dL) |
0 |
39.6 (36.4,43.0) |
44.2 (40.8,48.1) |
0.06 |
|
24 |
41.8 (38.2,45.4) |
42.0 (38.9,45.2) |
0.92 |
|
48 |
43.5 (39.6,47.5) |
40.0 (37.1,43.0) |
0.15 |
|
Serum creatinine (mg/dL) |
0 |
0.70 (0.67,0.73) |
0.77 (0.73,0.80) |
0.06 |
|
24 |
0.71 (0.69,0.73) |
0.72 (0.69,0.75) |
0.66 |
|
48 |
0.71 (0.68,0.74) |
0.69 (0.66,0.71) |
0.19 |
|
Serum potassium(mEq/L) |
0 |
3.95 (3.64, 4.26) |
4.12 (3.91, 4.34) |
0.34 |
|
24 |
4.18 (3.89, 4.47) |
3.87 (3.63, 4.11) |
0.09 |
|
48 |
4.45 (4.09, 4.80) |
3.63 (3.27, 3.99) |
0.01 |
|
Serum chloride(mEq/L) |
0 |
99.8 (97.4, 102.1) |
101.3 (98.9, 103.8) |
0.35 |
|
24 |
101.4 (98.9, 104.0) |
99.9 (97.5, 102.3) |
0.31 |
|
48 |
102.8 (99.7, 106.0) |
99.3 (96.6, 102.0) |
0.17 |
|
pH |
0 |
7.36 (7.34, 7.39) |
7.36 (7.34, 7.38) |
0.99 |
|
24 |
7.35 (7.32, 7.37) |
7.36 (7.34, 7.39) |
0.28 |
|
48 |
7.32 (7.30, 7.36) |
7.38 (7.35, 7.40) |
0.01 |
|
Blood sugar(mg/dL) |
0 |
104.3 (99.7, 110.1) |
99.3 (94.2, 103.9) |
0.17 |
|
24 |
108.8 (104.9, 112.8) |
99.6 (96.4, 102.8) |
<0.001 |
|
48 |
110.3 (107.3, 113.4) |
101.5 (98.1, 104.9) |
<0.001 |
|
¶ Mean (95% confidence interval), IF =Isotonic group, HF=
Hypotonic group; *N=30 at 0 hr and N=29 in both groups at 24 and
48 hr; # Independent Sample t-test for IF versus HF.
|
Serum sodium: Significant changes in sodium
levels were observed in both intervention groups (Table II).
In the IF group, mean serum sodium was constant over initial 24 hours;
thereafter, an increase was observed between 24 and 48 hours (4.3 meq/L,
95% CI: 0.1, 8.4 meq/L; (P=0.04). In HF group, there was a
significant decline in mean serum sodium level at 24 hours (6.5 meq/L,
95% CI: 3.5, 9.6 mEq/L; (P<0.001), and the decline persisted till
the end of study (4.9 meq/L, 95%CI: 1.9, 7.9 mEq/L; (P<0.001).
Serum osmolality: In IF group, serum osmolality
remained constant for the initial 24 hours and thereafter it increased
(mean increase at 48 hours 9.3 mos mol/L, 95% CI: 1.3, 17.2 mos mol/L;
P=0.02). In HF group, there was a decline starting at 24 hours
(14 mos mol/L, 95% CI: 8.1, 20 mos moL; P<0.001) and persisted
till the end of the observation period (11.7 mosmol/L, 95% CI: 5.7, 17.6
mosmol/L; P<0.001).
Blood urea/creatinine, serum potassium, blood sugar,
chloride and pH: Changes in blood urea and creatinine were not
significant, except for a significant (P<0.001) decline in
creatinine in HF group. There were no episodes of hypo - or hyper-kalemia.
Changes in blood sugar, serum chloride and pH in either group were not
significant.
Weight change: In IF group, there was
progressive fall in weight, and peak fall from baseline weight (2.6%,
95% CI: 1.9%, 3.3%) was at 48 hours (P<0.001). In HF group, there
was persistent rise in weight and maximum rise (2.8%, 95% CI: 1.0, 3.7%)
was noted at 48 hours (P<0.001).
Urine output: Change in urine output in IF group
was not significant (P=0.2) whereas in HF group, it increased
(0.15 mL/Kg/h, 95%CI: 0.06, 0.24 mL/kg/h; P<0.001). No episode of
oligouria or anuria occurred in either group.
Symptomatic dysnatremia and mortality:
There were no symptoms attributable to dysnatremia in either
intervention group. No mortality occurred in any of the group during the
observation period.
Discussion
The incidence of hyponatremia with the use of
hypotonic intravenous fluids has ranged from 7.6% to 57% [9-13]. We
observed unusually high incidence of 70% hyponatremia with the use of
hypotonic ‘standard volume’ maintenance fluid in a selected group of
non-surgical pediatric patients. In this study we tested the efficacy of
reduced volume isotonic maintenance fluid, and confirm significant
reduction in the risk of hyponatremia. However, in the absence of a
comparative IF group receiving unrestricted maintenance volume in this
study, it is difficult to assess the contribution of fluid restriction
versus higher fluid sodium, to the improved sodium levels in the
IF group.
Isotonic maintenance fluids have consistently been
shown to lower the incidence of hyponatremia [6,12-16]. Reducing the
volume of administered fluid has also been shown to decrease the risk of
hyponatremia in children with free-water excess [13]. Kannan, et al.
[13] have previously shown that hyponatremia is less common
with the use of isotonic saline in standard volume (1.72%) as well as
with reduced volume hypotonic saline solution (3.8%) when compared to
standard volume hypotonic fluid (14.3%). Young and Keeley [14], however,
reported that fluid type but not rate was significantly associated with
hyponatremia in surgical pediatric patients.
Similar to findings from our study, Saba, et al.
[16] also did not find a change in serum sodium in the first
12 hours in both intervention groups (isotonic or 0.45% NaCl). Thus
maximum risk of hyponatremia with use of either fluid is at 24 hours,
and warrants close clinical and laboratory monitoring. Similar to our
observations, Neville, et al. [12] observed that serum osmolality
remained constant in patients receiving isotonic fluids (0.9% NS),
whereas there was significant fall after 4 hours of fluid therapy in
those receiving 0.45% saline. Thus, continued administration of
restricted volume isotonic fluids beyond 24 hours may impose risk of
dehydration and hypernatremia.
Our study had certain limitations. First, the study
excluded all surgical patients as well as serious medical conditions
because of variability in their fluid requirements. In usual clinical
settings, these patients commonly require parenteral maintenance fluid
administration and are more prone to develop dysnatremia. Second, a
comparative IF arm, receiving full maintenance fluid would have been
useful to ascertain the influence of fluid restriction over and above
isotonic saline administration. Third, we did not measure urinary
electrolytes/osmolality and serum anti-diuretic hormone levels. These
would have been important for assessing volume status and free water
clearance. Lastly, since none of the patients in either group received
other fluids, it is difficult to extrapolate the results of this trial
to usual clinical scenario wherein patients often require deficit
replacements, partial maintenance fluid and sodium from other sources.
Further studies with a larger sample size and an additional control arm
using standard volume isotonic fluids may determine the overall benefit
and safety of volume reduction.
To conclude, intravenous isotonic fluid at the rate
of 60% of daily maintenance fluid requirement results in fewer episodes
of hyponatremia than standard volume maintenance hypotonic fluid during
48 hours of treatment without significant increase in the incidence of
hypernatremia. Continued administration of isotonic fluids beyond 24
hours, deserve close monitoring and modification of fluid therapy.
Contributors: AS: concept of study, data
collection, analysis, drafting and revision of manuscript; KA: concept,
design and supervision of the work; analysis of data; and drafting and
revision of manuscript for important intellectual content; SMA:
supervision of the work, data analysis and revision of manuscript. KA
will act as the guarantor.
Funding: None; Competing Interests:
None stated.
|
What is Already Known?
•
Standard volume maintenance
isotonic fluids reduce the incidence of hyponatremia in
comparison to hypotonic maintenance fluids in children.
What This Study Adds?
•
Reduced volume isotonic maintenance fluids results in fewer
episodes of hyponatremia in comparison to hypotonic maintenance
fluids in non-surgical pediatric patients.
•
The risk of hyponatremia is highest at 24 hours of
intravenous fluid administration, and requires close monitoring.
|
References
1. Holliday MA, Segar ME. Maintenance need for water
in parenteral fluid therapy. Pediatrics. 1957;19:823-32.
2. Anderson RJ. Hospital associated hyponatremia.
Kidney lnt. 1986;29:1237-47.
3. Arieff AI, Ayus JC, Fraser CL, Hyponatraemia and
death or permanent brain damage in healthy children. BMJ.
1992;304:1218-22.
4. Halberthal M, Halperin ML, Bohn D. Acute
hyponatremia in children admitted in hospital. BMJ. 2001;322:780-2.
5. Holliday MA, Friedman A, Segar ME, Chesney R,
Finberg L. Acute hospital induced hyponatremia in children: A
physiological approach. J Pediatr. 2004;145:584-7.
6. Montañana PA, Modesto i Alapont V, Ocón AP, López
PO, López Prats JL, et al. The use of isotonic fluid as
maintenance therapy prevents iatrogenic hyponatremia in pediatrics: A
randomized, controlled open study. Pediatr Crit Care Med. 2008;9:658-9.
7. Briassoulis G, Venkataraman S, Thompson AE. Energy
expenditure in critically ill children. Pediatr Crit Care Med.
2000;28:1166-72.
8. Choong K, Bohn D. Maintenance parenteral fluids in
the critically ill child. J Pediatr (Rio J). 2007; 83:S3-10.
9. Duke T, Mokela D, Frank D, Michael A, Paulo T,
Mgone J, et al. Management of meningitis in children with oral
fluid restriction or intravenous fluid at maintenance volumes: a
randomized trial. Ann Trop Pediatr. 2002;22:145-57.
10. Hoorn EJ, Geary D, Robb M, Halperin ML, Bohn D.
Acute hyponatremia related to intravenous fluid administration in
hospitalized children: An observational study. Pediatrics.
2004;113:1279-84.
11. Brazel P, McPhee IP. Inappropriate secretion of
antidiuretic hormone in postoperative scoliosis patients: The role of
fluid management. Spine. 1996;21:727.
12. Neville K, Verge C, Rosenberg A, O’Meara M,
Walker J. Isotonic is better than hypotonic saline for intravenous
rehydration of children with gastroenteritis: A prospective randomized
study. Arch Dis Child. 2006;91:226-32.
13. Kannan L, Lodha R, Vivekanandhan S, Bagga A,
Kabra SK, Kabra M. Intravenous fluid regimen and hyponatraemia among
children: A randomized controlled trial. Pediatr Nephrol.
2010;25:2303-9.
14. Yung M, Keeley S. Randomised controlled trial of
intravenous maintenance fluids. J Paediatr Child Health. 2009;45:9-14.
15. Choong K, Arora S, Cheng J, Farrokhyar F, Reddy
D, Thabane L, et al. Hypotonic versus isotonic maintenance fluids
after surgery in children: A randomized controlled trial. Pediatrics.
2011;128:857-66.
16. Saba TG, Fairbairn J, Houghton F, Laforte D,
Foster BJ. A randomized controlled trial of isotonic versus hypotonic
maintenance intravenous fluids in hospitalized children. BMC Pediatr.
2011;11:82.
|
|
|
 |
|

