|
|
|
Indian Pediatr 2011;48: 961-963 |
 |
Risk Factors For Prolonged Shedding of 2009
H1N1 Influenza Virus |
|
Yinghu Chen, Huiju Qiao, Chen Mei Zhang, Meiqin Tong and Shiqiang Shang
From the Division of Infection Disease, Zhejiang Key
Laboratory for Neonatal Disease, Children’s Hospital of Zhejiang
University Medical College, Hangzhou 310003, China.
Correspondence to: Shiqiang Shang, Professor, Head
of Division of Infection Disease, Children’s Hospital of Zhejiang
University Medical College, 57# Zhugan Lane, Xiacheng District, Hangzhou
310003, China.
Email: [email protected]
Received: October 21, 2010;
Initial review: November 04, 2010;
Accepted: February 22, 2011.
Published online: 2011 May 30.
PII: S09747559INPE1000356-2
|
|
Abstract
This retrospective study was conducted to estimate
the shedding of 2009 H1N1 virus and the risk analysis by review of
medical charts, laboratory and radiological findings of all inpatients
with confirmed pandemic influenza A (H1N1) at a provincial pediatric
hospital. A total of 41 cases attending the inpatient department
between 15 November, 2009 to 14 December, 2009 were included.
Prolonged and discontinuous shedding of 2009 H1N1 virus (median,
10days; range, 2 to 24 days) were detected by real-time RT-PCR. The
interval from onset of symptom to the start of oseltamivir therapy was
an independent risk factor for prolonged virus shedding.
Key words: 2009 H1N1 influenza; RT-PCR; Virus shedding.
|
|
T
he 2009 H1N1 influenza caused human infection in
Mexico and the United States in late April 2009, and subsequently spread
worldwide. Worldwide more than 214 countries and overseas territories or
communities have reported laboratory confirmed cases of pandemic influenza
H1N1 2009, including over 18,000 deaths [1].
The duration of virus shedding would provide important
knowledge for epidemiological control, antiviral therapy and infection
control measures. 2009 H1N1 influenza virus shedding in adults has been
reported to range from 1 to 28 days, and median length varied from 3 to 6
days [2-5]. The duration of novel influenza virus shedding was associated
with patients’ age, immunologic status, receiving anti-virus therapy and
viral resistant mutation [2-5]. To date, there is little research on
length of virus shedding and its risk factors in children.
Methods
This retrospective study was conducted by review of
medical charts, and laboratory and radiological findings of all children
admitted to the Children’s Hospital of Zhejiang University Medical College
with confirmed pandemic (H1N1) 2009. The study period was from 15
November, 2009 to 14 December, 2009. During this period all the children
admitted to the hospital with a febrile or respiratory illness were tested
for pandemic (H1N1) 2009 by real-time reverse transcriptase-polymerase
chain reaction with primers made by the CDC lab. A national guideline,
adapted from guidelines provided by the US Center for Disease Control and
Prevention was used to direct the surveillance, severity of illness,
diagnosis, and treatment of the disease. Patients whose first specimen was
collected prior to antiviral therapy were included in this analysis, and
their nasopharyngeal swab collection discontinued after one to three
consecutive negative results. The tests were done at a laboratory operated
under the auspices of the Chinese Center for Disease Control and
Prevention. The PCR products were sequenced for further confirmation with
the use of the BigDye Terminator, version 3.1 Cycle sequencing Kit
(Applied Biosystems) in accordance with the manufacturer’s instructions.
Specimens were collected from nasal pharyngeal swabs and had been
collected every one or two days since the pandemic (H1N1) 2009 was
confirmed. Epidemiological and clinical information collected were age,
gender, pre-existing medical conditions, severity of illness, date of
symptom onset, co-infections, specimen collection and antiviral therapy.
The Research Ethics Board at Children’s Hospital of Zhejiang University
Medical College approved the study design. Statistical analysis was
performed by binary logistic-regression analysis (Statistical package for
social sciences, 15th version). P value <0.05 was considered
significant.
Results
During the period from 15 November to 14 December 2009,
41 cases were enrolled in this study, of whom 10 (24%) were admitted to
ICU and 22 were male (54%). The median age was 34 months (range: 1 month
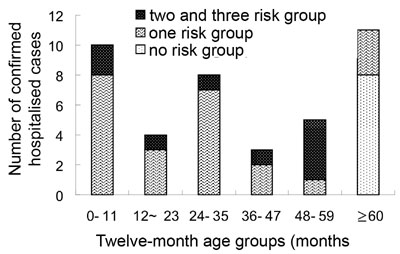
to 144 months). Thirty cases (73%) were under 59 months of age. Fig.1
shows the age-specific number for confirmed hospitalized cases of 2009
H1N1 influenza, by month age groups, by risk factors for complications,
including children younger than 5 years of age and those with underlying
medical conditions including: asthma (5 cases), neurological and
neurodevelopmental conditions (2 cases), chronic lung disease (0), heart
disease (1 case), blood disorders (4 cases), endocrine disorders (0),
kidney disorders (1 case), liver disorders (0), metabolic disorders (0),
and deficiencies in immune function due to disease or medication (4
cases). 4 cases, 3 cases, 1 case and 2 cases with prolonged viral shedding
had asthma, blood disorders, kidney disorders and neurological conditions,
respectively.
 |
|
Fig. 1. Age-specific cumulative number for
confirmed hospitalized cases of pandemic influenza A (novel H1N1),
by high risk factors for complications. |
Data on repeat RT PCR for novel H1N1 virus in
pharyngeal swabs were available for all cases. The mean, median days of
virus shedding were 11 days, 10 days respectively (range from 2 days to 24
days). For 6 ICU cases, we had the chance to monitor the viral shedding
after the first one or two times of negative result, 4 of them transiently
turned positive for one to two days, all of these four cases developed
severe complications and 3 of them had co-infections; suggesting the virus
shedding might be discontinuous. Twenty-one children (51%) received
antiviral therapy 7 days after onset of symptom or later. Table
1 shows the risk of viral shedding for ≥10 days. The binary
logistic-regression analysis revealed only the interval from symptom
onset to oseltamivir therapy was an independent risk factor for prolonged
virus shedding (odds ratio, 8.4; P=0.006).
TABLE I Risk of Viral Shedding for 10 Days or More
|
|
Length of viral
shedding (days) |
P
value * |
|
Variable |
≥10 d |
<10 d |
|
|
|
n=22 |
n=19 |
|
|
Age < 5y |
18 |
12 |
0.186 |
|
Male |
14 |
8 |
0.171 |
|
Immunodeficient |
3 |
1 |
0.762 |
|
Fever |
17 |
17 |
|
|
≥ 7 d Interval |
|
|
|
from symptom onset to
oseltamivir therapy |
17 |
4 |
0.006 |
|
ICU patients |
7 |
3 |
0.241 |
|
Pyretolysis |
|
|
|
|
≤ 24 hr after oseltamivir |
13 |
|
13 |
|
Clinical outcome |
|
|
|
|
Cure |
20 |
16 |
|
|
Improvement |
2 |
2 |
|
|
Death |
0 |
0 |
|
|
* Data are from
Binary logistic-regression analysis. Viral shedding was assessed on
the basis of the results of
Reverse-transcriptase-polymerase-chain–reaction testing. |
Discussion
At the late stage of the pandemic in Hangzhou city, the
majority of patients had shifted to young children, this prolonged virus
shedding in children was similar to the previous studies in seasonal
influenza virus infection, it could persist for up to 21 days [6], viral
load was found to be especially high in young children [7]. Children had
longer pandemic (H1N1) 2009 virus shedding than adults [3,4], which
provides information regarding virus-host inter-action. The interval from
onset of symptom to oseltamivir therapy was an independent risk factor for
prolonged shedding, similar to the finding reported by Cao, et al.
[3]. This prolonged virus shedding may be accounted for by delay in
receiving oseltamivir therapy, for the drug can markedly reduce the
replication of novel H1N1 virus in macrophage cells and dendritic cells
[8]. The odds ratio was higher in groups younger than 5 years old, male,
ICU patients and immunodeficiency patients, but there were no significant
difference on statistical analysis; the small sample size may be a reason.
The novel H1N1RT-PCR transiently turned positive after
it had become negative in the some patients; suggesting the virus shedding
was discontinuous. Co-infection and severe complication might be the
clinical features of discontinuous shedding. Although a positive result of
real-time RT-PCR testing does not necessarily indicate shedding of
infective virus, PCR is more sensitive than culture for viral detection
[4]. The extent of viral shedding would provide essential information in
developing further study, such as detecting viable shedding for designing
management polices in infection control.
Our study has several limitations. The sample size was
small, we neither tested the viral load, nor did virus culture. We also
did not test the resistant mutation strains.
Acknowledgments: Municipal Public Health
Outbreak Response Team, Departments of Public Health, the municipal Virus
Reference Laboratory, as well as hospital clinicians. We also thank
Professor Fangqi Gong, Meichun Xv, and statistician Jianfeng Liang.
Contributors: YC: design, data collection, and
drafting; HQ: patients care, and data collection; CZ: patient care; MT:
patients care; SS: supervisor and responsible for paper.
Funding: Natural Science Foundation of Zhejiang
Province (No. Y20110220).
Competing interests: None stated.
|
What This Study Adds?
• The interval from onset of symptom to the start
of oseltamivir therapy is an independent risk factor for prolonged
virus shedding of 2009 H1N1 influenza virus.
|
References
1. World Health Organization. Global Alert and Response
(GAR). Pandemic (H1N1) 2009 - update 106. Available from: http://www.who.int/csr/don/2010_06_25/en/index.html.
2. Fleury H, Burrel S, Balick Weber C, Hadrien R,
Blanco P, Cazanave C, et al. Prolonged shedding of influenza A
(H1N1)v virus: two case reports from France 2009. Euro Surveill. 14(49).
pii: 19434.
3. Cao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, et
al. Clinical features of the initial cases of 2009 pandemic influenza
A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507-17.
4. To KK, Chan KH, Li IW, Tsang TY, Tse H, Chan JF,
et al. Viral load in patients infected with pandemic H1N1 2009
influenza A virus. J Med Virol;2010, 82:1-7.
5. Witkop CT, Duffy MR, Macias EA, Gibbons TF, Escobar
JD, Burwell KN, et al. Novel Influenza A (H1N1) outbreak at the
U.S. Air Force Academy epidemiology and viral shedding duration. Am J Prev
Med. 2010;38:121-6.
6. Hall CB, Douglas RG Jr, Geiman JM, Meagher MP. Viral
shedding patterns of children with influenza B infection. J Infect Dis.
1979;140:610-3.
7. Osterlund P, Pirhonen J, Ikonen N, Rönkkö E,
Strengell M, Mäkelä SM, et al. Pandemic H1N1 2009 influenza A virus
induces weak cytokine responses in human macrophages and dendritic cells
and is highly sensitive to the antiviral actions of interferons. J Virol.
2010;84:1414-22.
8. World Health Organization Writing Group.
Non-pharmaceutical interventions for pandemic influenza, international
measures. Emerg Infect Dis. 2006;12:81-7.
|
|
|
 |
|

