|
|
|
Indian Pediatr 2011;48:
949-954 |
 |
Clinical Pulmonary Infection Score to Diagnose
Ventilator-associated Pneumonia in Children |
|
A Sachdev, K Chugh, M Sethi, D Gupta, C Wattal and G
Menon
From the Department of Pediatrics and *Department of
Microbiology, Sir Ganga Ram Hospital, Rajinder Nagar,
New Delhi 110 060, India.
Correspondence to: Dr Anil Sachdev, 63/12, 1st Floor, Old
Rajinder Nagar, New Delhi 110060, India. [email protected]
Received: May 15, 2010; Initial review: June 28, 2010;
Accepted: November 23, 2010.
Published online: 2011 March 15. PII: S097475591000411-1
|
Background: There is a need to validate and suggest
easy clinical method for diagnosis of ventilator-associated pneumonia (VAP)
in developing countries.
Objectives: To validate the use of simplified
Clinical Pulmonary Infection Score (CPIS) for the diagnosis of VAP.
Design: Prospective study.
Setting: Pediatric intensive care unit of a
tertiary care teaching hospital.
Subjects: 30 children receiving mechanical
ventilation for more than 48 hours and with simplified CPIS³6.
Methods: All patients underwent flexible
bronchoscopy to obtain bronchoalveolar lavage which was analyzed
quantitatively. Colony count≥104cfu/mL was considered reference
standard for definite VAP.
Results: Of the five variables used for simplified
CPIS, only patient’s temperature (P=0.013) and PaO2/ FiO2 ratio
were significant (P<0.001) to differentiate the presence of
definite VAP. Patients with definite VAP (BAL colony count ≥104cfu/mL)
had CPIS of 8.4 while in no definite VAP group it was 6.4 (P=
0.007). CPIS of 8 was found to have sensitivity of 80%, specificity 80%,
PPV 86.9%, NPV 70.5% and accuracy 80%. The area under Receiver operating
characteristic curve of CPIS against reference standard was 0.81± 0.069 (P=0.001).
Conclusion: Simplified CPIS is useful in patients
on mechanical ventilation to diagnose ventilator- associated pneumonia.
Key words: Bronchoscopy, Clinical pulmonary infection score,
India, Mechanical ventilation, Ventilator-associated pneumonia.
|
|
V
entilator-associated pneumonia (VAP)
is
an important problem in Pediatric
intensive care units (PICU). The
prevalence ranges from 3%-65% in PICUs in the USA [1]. The clinical
diagnosis of VAP is usually based on the presence of fever, leukocyte
counts, amount and character of tracheal secretions, and appearance of new
or persistence of radio-graphic infiltrates. However, these parameters
taken separately have limited diagnostic value [2]. The clinical criteria
for the diagnosis of VAP have repeatedly been criticized as inappropriate,
leading to over diagnosis or under diagnosis particularly in the setting
of Adult respiratory distress syndrome (ARDS) [3-5]. Despite these
limitations, the clinical criteria remain the starting point of diagnostic
evaluation of suspected VAP case. Pugin, et al. [6] combined the
above mentioned parameters with oxygenation (PaO2/FiO2)
and formed the Clinical Pulmonary Infection Score (CPIS) as a diagnostic
tool for pneumonia. The CPIS has been used in multiple studies on VAP in
adults [3,7-9] but limited data is available on pediatric cases [10]. This
prospective study was conducted to validate the use of CPIS to diagnose
VAP in pediatric patients.
Methods
During the 9 months period of the study, all patients
on mechanical ventilator for more than 48 hours by endotracheal tube (ETT)
were evaluated daily, in the morning, with simplified CPIS (with the help
of nursing charts) for the development of VAP and a score was assigned by
the designated investigator [9]. The chest X-ray and blood gas
analysis report done in the morning were used for scoring. The senior
nurses were trained to perform ETT suction. They were also instructed to
make a note of the nature and the amount of secretions by using -/+, + and
++ for few, moderate and large, respectively. To further ensure the
accuracy, the Critical care fellow or Senior resident on duty in the PICU
supervised this observation of the nurses. Patients with a CPIS score of ≥6 were included
in the study.
For all the enrolled patients, the following data were
recorded: age, gender, clinical presentation and date of suspicion of VAP,
and PICU admission and discharge. Time period of PICU stay prior to
initiation of and the duration of mechanical ventilation and the length of
PICU and hospital stays were also recorded. Chest radiographic findings at
the time of admission, on initiation of ventilation and at the time of
clinical suspicion of VAP were also recorded.
The enrolled patients were subjected to bedside
flexible bronchoscopy to obtain bronchoalveolar lavage (BAL). As per PICU
policy, all patients on ventilation receive continuous infusions of
midazolam and morphine. A bolus dose of vecuronium (0.1 mg/kg) was given
before starting bronchoscopy. All patients were monitored closely and
continuously during and after the procedure with multi-parameter monitor
and any cardio-pulmonary complications were recorded. No complications
were recorded in the present study.
Bronchoscopic bronchoalveolar lavage: The Olympus
BF type XP40 bronchoscope (Olympus Optical Co., Japan) with outer diameter
of 2.8 mm and a suction channel of 1.2 mm size was used to obtain lavage.
All patients were pre-oxygenated with 100% oxygen for 5-10 minutes. The
site for BAL was chosen according to the chest X-ray appearance of
localized infiltration or bronchoscopic appearance of inflammation or
purulent secretions. In the absence of above clues, BAL was performed in
the right lower or middle lobe. ETT suction was done just prior to
procedure. Bronchoscope was introduced through a swivel adapter connected
between ETT and ventilator circuit. In infants with 4.5 or less ETT size,
an appropriate size laryngeal airway mask was used for bronchoscopic
lavage [11]. Under visual control, the bronchoscope was advanced in the
direction of the chosen segment until a wedged position was achieved and
lavage was carried out with sterile saline. We used 3 mL for babies <5 Kg,
5 mL for children between 5-10 Kg, 7.5 mL for 11- 20 Kg and 10 mL for
patients >20kg. Lavage was sucked into a sterile mucus trap.
Microbiological methods: All BAL samples were
transported to the laboratory within 15 minutes and cultured within an
hour of collection. All samples were vortexed for a minute initially
before gram staining the smears. These preparations were studied for the
presence of squamous cells, polymor-phonuclear cells and the type of
microorganism present. Simultaneously, quantitative cultures using the
calibrated loop method were performed on common media such as blood agar,
chocolate agar and McKonkey’s agar using standard techniques [12,13].
Organisms were identified using automated Vitek-1 system (bioMerieux,
France). Microbio-logical examination for unusual organisms such as
Mycoplasma, Chlamydia, Pneumocystis carinii and viruses did not
form a part of this study.
The Institutional review board approval was obtained
for the study and informed consent for the bronchoscopic BAL was taken
from the parents. In accord with previous studies of quantitative
bacteriology of BAL cultures [1,14-17], a bacterial density of ≥10 4cfu
/mL was considered as positive for VAP and those episodes were referred to
as ‘Definite VAP’ episodes. The organisms isolated on blood culture were
compared with the organisms isolated from the BAL. The five parameters of
CPIS were compared between "Definite" and "No Definite" VAP patients using
independent sample t test and Chi-square test. Taking BAL colony
counts of ≥104
cfu/mL as the reference standard, the sensitivity,
specificity, positive and negative predictive values and accuracy were
calculated at various CPIS levels. The receiver operating characteristic
curve (ROC) was plotted and area under curve was also obtained. The
distribution pattern of the data was tested with D’Agostino- Pearson test
for normal distribution. P value less than 0.05 was considered
significant. SPSS 15 version statistical package was used for
analysis.
Results
There were a total of 267 ICU admissions during the
study period and 82 of these (30.7%) required mechanical ventilation (66
ventilated for more than 72 hours). Thirty patients with CPIS ≥6 were included in the
study (Table I). The mean duration of ICU stay up to the
initiation of mechanical ventilation was 1.4 days.
TABLE I Baseline Patient Characteristics (N=30)
|
Age median (range) |
6.5 years (1 mo -12 yrs) |
|
Males |
20 (66.5%) |
|
PRISM score* (range) |
13 ± 6 (0-34) |
|
Primary system involvement |
|
Central nervous system |
9 (30) |
|
Respiratory system |
6 (20) |
|
Septicemia |
5 (16.6) |
|
Diabetic ketoacidosis |
2 (6.6) |
|
Postoperative status |
2 (6.6) |
|
Trauma |
2 (6.6) |
|
Miscellaneous |
4 (13.3) |
|
Multiorgan dysfunction |
15 (50) |
|
Use of steroids |
4 (13.3) |
|
Immunosuppressed state |
2 (20) |
|
Use of H2 blockers |
9 (30) |
|
Chest X-ray at admission |
|
Normal |
21 (70) |
|
ARDS |
3 (10) |
|
Others |
6 (20) |
|
Chest X-ray at initiation of MV |
|
Normal |
14 (44.6) |
|
ARDS |
9 (30) |
|
Others |
7 (23.3) |
|
*mean ±SD; Figures
in parentheses indicate percentage; ARDS: acute respiratory distress
syndrome; MV: Mechanical ventilation. |
All study cases were receiving antibiotics and the mean
duration of therapy prior to development of VAP was 10.1 days. The median
duration of ventilation before the clinical diagnosis of VAP was 9 days
(range 3-60 days). The median total duration of MV was 16 days (range 7 to
120 days) while median stay in PICU was 20 days (range 9 to 124 days). The
mean duration of MV was significantly higher in children less than 1 year
as compared to older children (55 vs 20.2 days, P=0.001).
Microbiological results: Eighteen blood cultures
were positive. The most common organism cultured was Pseudomonas
aeruginosa (43.6%). Other organisms obtained were Acinetobacter
baumannii, Enterobacter sps, methicillin resistant Staphy-lococcus aures (MRSA)
and Candida albicans. Eleven (60%) blood culture results were
concordant with the organism yielded from BAL in colony counts of ≥104
cfu/mL. Twenty one out of 30 BAL samples yielded
positive cultures. However, only 19 samples yielded organisms with a
colony count of ≥104
cfu/ mL. Micro-organisms cultured included
P.aeruginosa (9), A. baumannii (5), K. pneumoniae (4),
Methicillin resistant S. aureus (2), P. mirabilis (1),
and Enterobacter species and Candida sepsis (3
each). In six samples, polymicrobial growths were obtained. In three
samples, Candida sps grew along with Pseudomonas aeruginosa,
Klebsiella pneumo-naie and Acinetobacter baumannii.
Table II Comparison of Individual Variables of Simplified CPIS in Patients With Definite
and No Definite Ventilator-associated Pneumonia.
|
Variable |
Definite |
No Definite |
P |
|
|
VAP (n=19) |
VAP (n=11) |
value* |
|
TemperatureºC |
39.2 ± 0.72 |
38.5 ±0.8 |
0.01 |
|
TLC (mm3) |
18960±7094 |
16386±6296 |
0.2 |
|
PaO2/ FiO2 |
155±41.6 |
234±69 |
0.0 |
|
Tracheal secretions |
|
Scanty |
0 |
1 |
|
|
Moderate |
8 |
3 |
0.3 |
|
Profuse |
11 |
7 |
|
|
Chest X-ray |
|
Collapse |
8 |
6 |
|
|
Consolidation |
5 |
2 |
|
|
ARDS |
3 |
1 |
0.8 |
|
B/L haziness |
2 |
2 |
|
|
Cavitatory lesion |
1 |
0 |
|
|
CPIS |
8.48± 1.2 |
6.8 ± 1.2 |
0.007 |
|
*Temperature, TLC and PaO2/FiO2
compared using t test and
chest X-ray and tracheal secretions compared by Chi-square test; VAP
ventilator- associated pneumonia; TLC- total leucocyte count;,
ARDS:acute respiratory distress syndrome; CPIS" clinical pulmonary
infection score. |
Simplified clinical pulmonary infection scores: The
individual variables of CPIS were compared between patients with definite
and no definite VAP (Table II). None of the patient had
normal chest X-ray at the time of enrolment. The higher temperature
of patient (39.2 ± 0.7 vs 38.5 ± 0.8, P = 0.01) and low PaO2/FiO2
ratio (155.5 ± 41.6 vs 234 ± 69, P < 0.001) were significant
variables for the diagnosis of VAP. A value of 8 on the CPIS was found to
have the best accuracy with a sensitivity and specificity of 80% each (Table
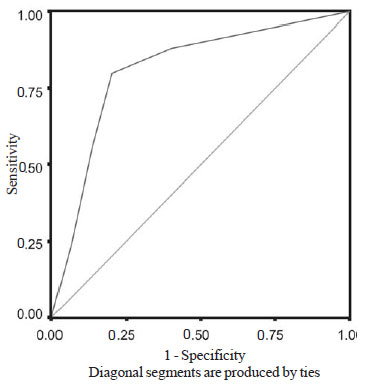
III). Area under the ROC curve was 0.812 (P < 0.001) (Fig.
1).
TABLE III Operative Indices of Simplified Clinical Pulmonary Infection Score for the Definite
Diagnosis of Ventilator-associated Pneumonia
|
CPIS |
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
|
6 |
100 |
0 |
62.5 |
0 |
62.5 |
|
7 |
88 |
60 |
78.5 |
75 |
77.5 |
|
8 |
80 |
80 |
87 |
70.5 |
80 |
|
9 |
61 |
87 |
87.5 |
54 |
67.5 |
|
10 |
24 |
93 |
85.7 |
42 |
50 |
CPIS: Clinical pulmonary infection score; PPV: Positive predictive value, NPV: Negative predictive value.
|
 |
|
Fig. 1 Receiver operating characteristic curve of clinical
pulmonary infection score.
|
Discussion
In this study, we have evaluated the clinical diagnosis
of VAP assessed by simplified CPIS using bronchoscopic BAL culture as the
reference standard. In the present study, individual variables used in
CPIS were also compared in patients with definite VAP and with no definite
VAP. Presence of fever and PaO2/FiO2
ratios were significantly different in two groups.
Pugin, et al. [6] found significant difference
of temperature in adult patients with or without pulmonary infection,
while other studies found presence of fever as a poor diagnostic marker,
especially in ARDS patients [3,4,7]. The presence of fever may be
explained by mechanisms other than pneumonia in patients on ventilator.
Meduri, et al. [18] found that only 42% of patients with fever and
pulmonary infiltrates had pneumonia. Leucocytosis and purulent tracheal
secretions had good sensitivity (77% and 69%, respectively) but poor
specificity (58% and 42%, respectively) in a study by Fabergus, et al.
[3]. It is quite obvious that leucocytosis may be induced by a variety of
causes in a critically ill patient [3]. The high false positivity of
purulent tracheal secretions is explained by the presence of purulent
bronchitis in almost all patients particularly during prolonged MV. The
negative results may be due to the peripheral location of pneumonia and
impaired clearance of secretions [19] .
In the present study, chest X-ray was positive
in all the patients at the time of enrolment. Similar observation was
reported by Andrews, et al. [4] but only 57% had histological
evidence of pneumonia. While Fabergus, et al. [3] found
radiographic infiltrates as most sensitive (92%) clinical parameter for
diagnosing VAP, the rate of false positive results was as high as 67%.
This high false positivity may result from other causes mimicking VAP such
as atelectasis, pulmonary infarction, alveolar hemorrhage and ARDS.
As per Pugin, et al. [6], a CPIS >6 was
associated with high likelihood of pneumonia (sensitivity 93%, specificity
100%). The disadvantage with this score is dependence on tracheal aspirate
gram stain and culture results and so the waiting period of 24 to 48 hours
for the clinical diagnosis of VAP [9,20]. In the present study, simplified
CPIS was used [9]. This score does not include any laboratory dependent
variables. The maximum possible score is 10. Based on the previous studies
patients with CPIS ≥6
were enrolled in the present study [3, 6-10, 21]. Pugin, et al. [6]
did not find any positive bronchoscopic BAL in patients with CPIS<6. The
likelihood ratio of detecting pneumonia was 1.46 and 0.68 in patients with
CPIS ≥6 and <6,
respectively [2]. In another study, CPIS >6 virtually ruled out other
causes of pulmonary infiltrates in ICU patients [22].
The mean CPIS was significantly high in patients with
definite VAP (P=0.007). CPIS has been studied in many adult studies
as a diagnostic tool [3,6]. Results of these studies are conflicting. High
sensitivity (93%) and high specificity (100%) of CPIS >6 were reported
[6]. Papazian, et al. [7] used CPIS for selection of cases to test
the diagnostic accuracy of bronchoscopic and nonbronchoscopic techniques.
CPIS at the threshold of 6 achieved an accuracy of 79%, a sensitivity of
72% and a specificity of 85%.The individual variables of CPIS were not
significantly different while the composite CPIS score was significantly
higher in the VAP group than in the non-VAP group. Similar results were
not reported in other adult studies [2,3]. The only pediatric study
evaluating CPIS had enrolled 15 mechanically ventilated children. In 14
patients CPIS was more than 6 at the time of diagnosis of VAP with
positive predictive value of 93% [10].
Serial CPIS and its components have been used
previously to assess the treatment response in prospective studies.
Progressively falling CPIS and rising PaO2/FiO2
ratio distinguished survivor from non-survivors [9]. CPIS had also
identified patients requiring antibiotic therapy and so reduced the cost
of therapy [22].
There is an important limitation with the use of CPIS.
All the elements of CPIS are given equal weighting. In other words, new
lobar infiltration or deteriorating PaO2/FiO2
is of greater importance to the clinician than leukocytosis, which is
non-specific. In clinical practice, CPIS has utility as a diagnostic tool
to identify patients with high probability of pneumonia requiring definite
diagnostic procedure and to evaluate the clinical response to therapy
[23].
Contributors: AS: concept, analysis and manuscript
preparation; KC: Critical review of manuscript; MS & DG: Data collection
and compilation; SW: Microbiological support; GM: Statistical analysis.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• Clinical pulmonary infection score (CPIS) is an easy
composite score.
What This Study Adds?
• A simplified CPIS is helpful to diagnose
ventilator-associated pneumonia in children in the PICU and to
initiate a definite diagnostic procedure.
|
References
1. Gauvin F, Dassa C, Chaibou M, Proulx F, Farrell CA,
Lacroix J. Ventilator-associated pneumonia in intubated children:
Comparison of different diagnostic methods. Pediatr Crit Care Med.
2003;4:437-43.
2. Fartoukh M, Maitre B, Honore S, Cerf C, Zahar JR,
Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation. The
clinical pulmonary infection score revisited. Am J Respir Crit Care Med.
2003;168:173-9.
3. Fabergas N, Ewig S, Torres A, El-Ebiary M, Ramirez
J, Bellacasa de la JP, et al. Clinical diagnosis of ventilator
associated pneumonia revisited: comparative validation using immediate
post-mortem lung biopsies. Thorax. 1999;54:867-73.
4. Andrew CP, Coalson JJ, Smith JD, Johanson WG Jr.
Diagnosis of nosocomial bacterial pneumonia in acute, diffuse lung injury.
Chest. 1981;80:254-8.
5. Torres A, El-Ebiary M, Padro L, Gonzalez J,
Bellacasa de la JP, Ramirez J, et al. Validation of different
techniques for diagnosis of ventilator-associated pneumonia: Comparison
with immediate postmortem pulmonary biopsy. Am J Respir Crit Care Med.
1994;149:324-31.
6. Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew
PD, Suter PM. Diagnosis of ventilator-associated pneumonia by
bacteriologic analysis of bronchoscopic and nobronchoscopic "blind"
bronchoalveolar lavage fluid. Am Respir Crit Care Med. 1991;143:1121-9.
7. Papazian L, Thomas P, Garbe L, Guignon I, Thirion X,
Cherral J, et al. Bronchoscopic and blind sampling techniques for
the diagnosis of ventilator-associate pneumonia. Am J Respir Crit Care.
1995;152:1982-91.
8. Flanagan PG, Findlay GP, Magee JT, Ionesue AA,
Barnes RA, Smithies MN. The diagnosis of ventilator-associated pneumonia
using non-bronchoscopic, non-directed lung lavages. Intensive Care Med.
2000;26:20-30.
9. Luna CM, Blanzaco D, Niedermn MS, Matarucco W,
Baredes NC, Desmery P, et al. Resolution of ventilator-associated
pneumonia: Prospective evaluation of the clinical pulmonary score as an
early clinical predictor of outcome. Crit Care Med. 2003;31: 676-82.
10. Grasso F, Chidini G, Napolitano L, Calderini E.
Ventilator- associated pneumonia in children: evaluation of clinical
pulmonary infection score in monitoring the course of illness. Crit Care.
2004;8:209.
11. Nassbaum E, Zagnoev M. Pediatric fiberoptic
bronchoscopy with laryngeal mask airway. Chest. 2001;120:614- 6.
12. James L, Hoppe- Bauer JE. Processing and
interpretation of lower respiratory tract specimen. In: Clinical
Microbiological Procedures Handbook. Isenberg HD (Ed) American Society of
Microbiology. Washington DC 1992. p. 1.15.1-1.15.8.
13. Clinical and Laboratory Standards Institute.
Performance standards for antimicrobial susceptibility testing, document M
100-S16. 2006. Clinical and Laboratory Standards Institute, Wayne, PA.
14. Chastre J, Fagon JY. Ventilator associated
pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
15. Elward AM. Pediatric ventilator- associated
pneumonia. Pedaitr Infect Dis J. 2003; 22:445-6.
16. Pingleton S K, Fagon JY, Leeper K V Jr. Patient
selection for clinical investigation of ventilator associated pneumonia.
Criteria for evaluating diagnostic techniques. Chest. 1992;102:553-6.
17. Labenne M, Poyart C, Ramband C, Goldfarb B, Pron B,
Jouvet P, et al. Blind protected specimen brush and bronchoalveolar
lavage in ventilated children. Crit Care Med. 1999;27:2537- 43.
18. Meduri GU, Mauldin GL, Wunderink RG, Leeper KV,
Jones CB, Tolley E, et al. Causes of fever and pulmonary densities
in patients with clinical manifestations of ventilator- associated
pneumonia. Chest. 1994;106: 221-35.
19. Rouby JJ, Martin de Lassale E, Poete P, Nicholas MH,
Bodin H, Jarlier V, et al. Nosocomial bronchopneumonia in critically ill.
Am Rev Respir Dis. 1992;146: 1059-66.
20. Court A, Garrard C, Crook D, Bowler I, Conolon C,
Peto T, et al. Microbiological surveillance of the lungs using
non-directed bronchial lavage. Q J Med. 1993;86:635-48.
21. Singh N, Rogers P, Atwood CW, Wegner MM, Yu VL.
Short course of empiric antibiotic therapy for patients with pulmonary
infiltrates in the intensive care unit. A proposed solution for
indiscriminate antibiotic prescription. Am J Respir Crit Care Med.
2000;162:505-11.
22. Singh N, Falestiny MN, Rogers P, Reed MJ, Pularski
J, Norris R, et al. Pulmonary infiltrates in the surgical ICU:
Prospective assessment of predictors of aetiology and mortality. Chest.
1998;10:1129-36.
23. Hubmayr RD. Statement of the 4th International Consensus Conference
in Critical Care on ICU-acquired Pneumonia- Chicago, Illinois, May 2002.
Intensive Care Med. 2002;28:1521-36.
|
|
|
 |
|

