|
|
|
Indian Pediatr 2010;47: 1066-1068 |
 |
Sinus Node Paucity in Hyperekplexia |
|
S Ozkiraz, Z Gokmen, UA Orün*, and F Alehan†
From Departments of Neonatology, *Pediatric Cardiology,
†Pediatric Neurology,
Medical School of Baskent University, Ankara, Turkey.
Correspondence to: Dr Servet Ozkiraz, Department of
Neonatology,
Medical School of Baskent University, Ankara, Turkey.
Email: sozKiraz@yahoo.com
Received: March 30, 2009;
Initial review: June 15, 2009;
Accepted: August 18, 2009.
|
We report a newborn with hyperekplexia and uncontrolled tonic spasms
which did not respond to intravenous phenobarbitone and phenytoin, and
midazolam infusion. Serum biochemistry, electrocardiography,
electroencephalography, lumbar puncture and neuroimaging were normal.
Continous cardiac monitoring revealed that tonic spasm episodes were
accompanied by sinus node paucity and severe bradycardia. Duration and
number of tonic spasm episodes decreased with clonazepam therapy, and
she was discharged. At 4 months of age sudden infant death occured.
Sudden infant death could be related to the paucity of sinus node.
Cardiac pacemaker implantation should be considered even if the medical
treatment is successful.
Key words: Hyperekplexia, Neonate, Seizures, Sinus node.
|
|
Hyperekplexia
or Startle disease is a rare, sporadic or autosomal dominant disorder that
with variable expression(1). Most patients present in the neonatal period;
clinical picture is characterized by myoclonic jerks, increased muscle
tone, tonic spasms, generalized hyperreflexia and severe apnea without
concomitant discharges on electroencephalography (EEG). This disorder is
frequently misdiagnosed as convulsion in neonatal period.
Life-threatening tonic spasm episodes associated with
severe apnea and bradycardia may occur. These episodes may result in
sudden death(2-5). Although cardiac irregularities - such as bradycardia,
tachycardia, and complete heart block, have been demonstrated in
hyperekplexia, sinus node paucity has not been reported. We report a
newborn with the sporadic form of hyperekplexia who had episodes of tonic
spasms accompanied by sinus node paucity and severe bradycardia.
Case Report
A girl born at term by cesarian section, with a birth
weight of 3000 g had Apgar score of 7 and 8 at 1 and 5 minutes. Her
parents were unrelated and she was the first child. There was no history
of prenatal alcohol or drug use. The child was noticed to have exaggerated
startle and Moro reflex during first few hours of life. This was
associated with generalized hypertonicity, hyperreflexia, and tonic
spasms, mimicking tonic seizures lasting up to 5-10 seconds and leading to
feeding difficulties. Phenobarbital, phenytoin and midazolam infusion were
started, in that order, to control the seizures. Serum amino and organic
acids, serum lactate and pyruvate concentrations, lumbar puncture, EEG and
cranial magnetic resonance imaging and cranial computed tomography were
normal. Since there was no resolution of symptoms and spasms continued,
the infant was transferred to our hospital on postnatal day 25. Family
history was negative for epilepsy and symptoms of hyperekplexia.
Examination on admission revealed an alert infant with
exaggerated startle reflex, generalized hypertonicity and hyperreflexia.
She was normal at sleep. Nose tapping resulted in retraction of the head,
followed by flexor spasm of all extremities and exaggerated startle
lasting up 10 seconds, without habituation. The hemogram, serum
electrolytes, ammonia, lactate dehydrogenase level, serum lipid levels,
serum lactate and pyruvate, lumbar puncture, cultures from blood, urine,
cerebrospinal fluid, multiple electro-encephalographies (EEG), brain
computed tomography and magnetic resonance imaging, electromyography and
echocardiography were all normal. Midazolam, phenobarbital and phenytoin
were of no benefit and were discontinued and clonazepam was started at a
dosage of 0.1 mg/kg/day. Flexion of head and lower extremities toward the
trunk was beneficial.
Some stiffening episodes were accompanied by
bradycardia and desaturation of arterial oxygen saturation. Continuous
cardiac monitoring demons-trated an average baseline heart rate of 138/min
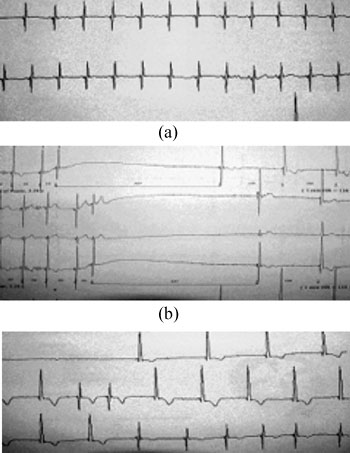
(range: 114-165/min) (Fig.1a). Episodes of stiffening
spasms followed by bradycardia leading to paucity of sinus node nearly to
3.6 seconds, followed by a junctional atrial escape rhythm at 54-65/min.,
were recorded 13 times in a 24-hour period (Fig. 1b,c).
Bradycardia as low as 20/min during episodes was associated with
desaturation of arterial oxygen measured by pulse oxymetry (SaO 2
50%; baseline oxygen saturation: 95-100%). Not all bradycardic episodes
were associated with apnea. A cardiac pacemaker insertion was discussed
but because of a decrease in number and duration of these episodes after
clonazepam therapy, cardiac pacemaker was not inserted. She was dicharged
on day 65 with clonazepam and orogastric tube feeding. At age of 4 months,
sudden infant death occurred.
 |
|
(C) |
|
Fig.1 Electrocardiogram rhythm strip from
Holter monitor. (a) baseline rate of 138/min; (b) onset of tonic
spasm associate with sinus node paucity duration of 3.6 seconds; (c)
junctional atrial escape rhythm with rate of 54-65/min and rate
returns to 135/min. |
Discussion
Hyperekplexia is a rare and generally benign disorder.
Shahar and Raviv(6) reported 39 children diagnosed as sporadic major
hyperekplexia presenting at an average of 3.3 months and treated with low
doses of oral clonazepam. All of them recovered. But sometimes
hyperekplexia may become life-threatening, leading to sudden infant death
if not promply diagnosed and treated accordingly.
The underlying pathophysiology of tonic spasms in
hyperekplexia is controversial. In 30% of patients with familial
hyperekplexia several mutations in the alpha-1 subunit of the glycine
receptor linked to chromosome 5q33-35 have been reported(7). Family
history was negative for familial hyperekplexia, and we did not
investigate the mutation analysis of glycine receptor. As an inhibitory
neurotransmitter, glycine plays an important role in the neuronal
regulation of muscle tone in the brain stem and spinal cord. Once released
from the presynaptic vesicles, glycine binds to the
a-1
subunit of glycine receptor, which causes the channel to open for Cl-,
thus hyperpolarizing the postsynaptic cell. Selective blockade of
glycinergic inhibition by strychnine or by tetanus toxin results in
excessive startle, and massive spasms of the trunk and limbs.
Correspondingly, mutations of the
a1
subunit of the glycine receptor cause a variety of dysfunctions of the Cl-
channel, therefore, is regarded as a channelopathy(8). Decreased
concentrations of the inhibitory
a-aminobutyric
acid have been also detected in cerebrospinal fluid in infants with
hyperekplexia(9). In addition, response to central
a-aminobutyric
acid-benzodiazepines agonist such as clonazepam implies a possible role
for overflow of abundant excitatory bioamines in the pathophysiology of
hyperekplexia(6). The underlying pathology of sudden infant death
syndrome, apnea, and cardiac irregularities such as bradycardia and
complete heart block in hyperekplexia is speculated as brainstem-generated
autonomic dysfunction(4,9).
Cardiac irregularities; such as bradycardia,
tachycardia, and complete heart block, have been reported in
hyperekplexia(3,9,10). McAbee, et al.(10) reported a newborn with
tonic spasm episodes associated with prolonged apnea and complete heart
block requiring the implantation of permanent cardiac pacemaker. Sinus
node paucity has not been reported in hyperekplexia. Sinus node paucity
occurs in infants with cardiac structural abnormalities, especially in
sinus venosus defects, and after atrial surgery. Our infant had no
evidence of structural cardiac disease. Cardiac pacemaker can be effective
in minimizing the risk of sudden death from paucity of sinus node(11). A
cardiac pacemaker insertion was discussed but because of a decrease in
number and duration of these episodes after clonazepam therapy, cardiac
pacemaker was not inserted. She was discharged on day 50 with clonazepam
and orogastric tube feeding, sudden infant death occured at 4 months of
age. Close cardiac monitoring and cardiac pacemaker implantation could
have averted the sudden infant death.
Contributors: All the authors were involved in all
aspects of manuscript preparation.
Funding: None. Competing interests: None
stated.
References
1. Suhren O, Bruyn GW, Tuynman JA. Hyperekplexia: a
hereditary startle syndrome. J Neurol Sci 1966; 3: 577-605.
2. Kurcyznski TW. Hyperekplexia. Arch Neurol 1983; 40:
246-248.
3. Vigevano F 1989, Capua MD, Bernadina BD. Startle
disease: An avoidable cause of sudden infant death. Lancet 1989; 1: 216.
4. Giacoia GP, Ryan SG. Hyperekplexia associated with
apnea and sudden death syndrome. Arch Pediatr Adolesc Med 1994; 148:
540-543.
5. Nigro MA, Lim HC. Hyperekplexia and neonatal death.
Pediatr Neurol 1992; 31: 63-68.
6. Shahar E, Raviv R. Sporadic major hyperekplexia in
neonates and infants: clinical manifestations and outcome. Pediatr Neurol
2004; 31: 30-34.
7. Tijssen MAJ, Vergouwe MN, Gert van Dijk J, Rees M,
Frants RR, Brown P. Major and minor form of hereditary hyperekplexia. Mov
Disord 2002; 17: 826-830.
8. Meinck HM. Startle and its disorders.
Neurophysiologie Clinique 2006; 36: 357-364.
9. Dubowitz LMS, Bouza H, Hird MF, Jaeken J. Low
cerebrospinal fluid concentration of free gamma-aminobutyric acid in
startle disease. Lancet 1992; 340: 80-81.
10. McAbee GN, Kadakia SK, Sisley KC, Eegt R, Delfiner
JS. Complete heart block in nonfamilial hyperekplexia. Ped Neurol 1995;
12: 149-151.
11. Maginot KR, Mathewson JW, Bichell DP, Perry JC.
Applications of pacing strategies in neonates and infants. Prog Ped
Cardiol 2000; 11: 65-75.
|
|
|
 |
|

