|
With
early aggressive treatment and robust supportive care, more than 7 out of
10 children with cancer in the developed world are cured(1). In such a
setting, toxicity-related death and relapse, although less common,
nevertheless are the main causes of treatment failure(2). However, 80% of
the approximately 200,000 new cases of childhood cancer each year
worldwide live in resource-limited countries(3). In contrast to what is
seen in the developed world, failure of treatment of childhood cancer is
still a common occurrence worldwide with refusal (non-initiation) and
abandonment (non-completion) of treatment often exceeding all other causes
of failure(2,4,5). In the developed world, any refusal or abandonment is
likely to lead to health and social services intervening and they may even
take court action to ensure that the child receives treatment. Such state
support and intervention is non-existent in large parts of the world,
including in India, so that treatment refusal and abandonment remain
common events. In a recent study from a tertiary healthcare establishment
in India, of 762 children with acute lymphoblastic leukemia, 30% refused
and another 15% abandoned treatment(6). As progress is being made to
reduce infection-related childhood deaths in India, it should no longer be
acceptable to allow children with cancer who have the potential for cure
with appropriate treatment, to be ignored when treatment abandonment
occurs.
In our efforts to improve the outlook of this chronic
but largely curable childhood disease, it is essential that we understand
and address the problem of treatment refusal and abandonment. It was
previously reported that this problem was wide-spread across the
developing world(7). The magnitude of refusal/abandonment was difficult to
estimate reliably as it varied between countries, the type of healthcare
system and type of cancer. In India, available data from tertiary centers
shows abandonment rates varying from 17- 62%(7). Even this figure is
likely to be an under-estimate as the bulk of the childhood cancer
patients are provided care by smaller healthcare establishments scattered
across the country. This would imply that, of the estimated 40,000-50,000
annual new cases of childhood cancer in India(8,9), the majority would not
be adequately treated and consequently die of their disease.
Even lesser has been our understanding of the causes of
treatment refusal and abandonment, and of the possible solutions. In 2003,
Metzger, et al.(4) had shown that abandonment of treatment in
children with acute lymphocytic leukemia (ALL) in Honduras was associated
with prolonged travel time to the treatment facility (>5 hr) and age
younger than 4.5 years but not with patient sex or ALL risk group(4).
Following this, Howard, et al.(2), in their seminal paper in
2004(2) showed that a multi-pronged and sustained approach involving
training of doctors and nurses, transfer of diagnostic and therapeutic
protocols, improving supportive care, financial aid, and involvement in
research projects resulted in impressive reductions in abandonment
(besides decrease in relapse and mortality rates and improvement in
five-year survival). In the last four years, more evidence of the
mechanism and interplay of different factors has begun to emerge from
across the world and herein we summarize these findings. Research from
India on this topic is still conspicuous by its absence.
Understanding the Causes
Age - Metzger, et al.(4) first reported an
association of abandonment with age less than 4.5 years in a cohort of
children with ALL(4). However, they could not explain this finding and
suggested that it was a confounder for some other association e.g family
size or lack of extended family support. Other studies have subsequently
not found such a relation between age and treatment
refusal/abandonment(10,11).
Gender - Gender of the child was also not found to
be associated with differences in abandonment rates in Central
America(4,10) and Indonesia(11). However, in a follow-up survey from North
India of those who abandoned treatment, 28% of parents reported that the
patient being a female influenced their decision(12). Gender, like age, is
a demographic variable, but any variation in behaviour based on gender can
also be a reflection of societal prejudices. Gender bias by parents when
seeking healthcare for children or for cancer registration is well
documented(13,14). Thus, it would not be unexpected to find a similar bias
when analyzing refusal/abandonment rates. Further investigation of the
extent of this bias is required.
Biology of the disease - One might expect that
childhood tumors requiring less intensive therapy (e.g. low risk ALL,
Hodgkin lymphoma, Wilms tumor) would have lower abandonment rates compared
with those cancers needing more intensive treatment with consequent higher
toxicity (e.g. high risk ALL, AML, high risk neuroblastoma) and cost.
There was a suggestion to this effect in our previous review(7). However,
nearly 90% of children with Wilms' tumor in Sudan refused or abandoned
treatment, thus illustrating that overall economic, social and political
factors are probably more significant than type of treatment itself(15).
In a recent study from El Salvador, abandonment rates were not different
between leukemias/lymphomas, CNS tumors and other solid tumors(10).
Abandonment rates were not found to be associated with the risk grouping
of children with ALL(4,11) or with length of protocol(10). Additional
research is needed before definitive conclusions can be drawn on the
impact of tumor type/biology of the disease on abandonment.
Treatment-related factors - While there is no clear
association between type of tumor and abandonment, it has been shown in
several studies that abandonment rates for ALL are highest during the
early phase induction(4,5,11). This may be related to multiple factors
acting singly or in combination. These include treatment-related toxicity,
painful procedures performed with inadequate analgesia and sedation,
inadequate communication provided by health care providers, predetermined
health beliefs of parents, and lack of finances. Although these factors
are relevant during the entire treatment, their role is amplified during
the initial intense phase of therapy. Studies have consistently shown that
adverse effects of treatment including painful procedures are of major
concern to parents, often contributing to abandonment(5,11,12,16). The
standard practice of using short-acting general anesthesia for bone marrow
aspirations/biopsies and lumbar punctures is uncommon in the developing
world. The unease of parents is also reflected in their health seeking
behaviour after they abandon treatment. The majority of them seek
complementary and alternative medical treatment, often citing lack of
adverse effects as a reason compared with modern chemotherapy(11,16,17).
Communication issues and attitude of health care
providers - Closely linked to the treatment related toxicity, is the
ability of the healthcare provider to communicate effectively with the
parents and the child. Clear and detailed information given repeatedly to
the parents is vital for them to understand the disease, its treatment and
the effects (beneficial and adverse) of treatment. This is particularly
important during the initial part of treatment and in a setting where a
majority of parents may believe in the inevitable "fatality" of
cancer(11,18). This belief is often based on a parent's experience of
witnessing a close adult family member or friend who had succumbed to
cancer. Only through repeated counseling can parents understand the
necessity of continuing and completing treatment after the cancer has
apparently long "disappeared". Resources and staff are often stretched in
providing care in developing countries. In such a setting, doctors can
come across as impatient, busy or irritated, which makes parents hesitant
in asking for information(16,18,19). In children with ALL, Mostert, et
al.(5,20) partly attributed the variation in abandonment rates between
children of prosperous parents (2%) and poor parents (47%) to the
differences in the quality and quantity of communication given and
individualized attention offered by healthcare providers.
Financial burden - Perhaps the single most
important factor that underpins all others is the financial resource of
the family. In developing countries, social and economic support from the
state is either non-existent or inadequate, and medical insurance mostly
absent(21). The burden of the cost of treatment falls mostly, if not
entirely, on the family of the child. Parents consistently report
financial burden as the main reason for abandonment and there is variation
in abandonment rates between children of prosperous parents and poor
parents(5,12). Not surprisingly, monthly income of the family has been
shown to be significantly related to abandonment rates(10). As shown in
Fig. 1, the cost of therapy is more than simply the sum of the
cost of investigations and treatment. Cost of transport is also a major
concern for parents and contributes to abandonment(11,12). Travel time to
treatment center has been shown to be significantly associated with
abandonment rates in Honduras(4). An indirect impact is also in the form
of loss of employment and income as well as incurring debts for the family
as a relative (and a key income generator) has to stay with the
child(11,18,19).
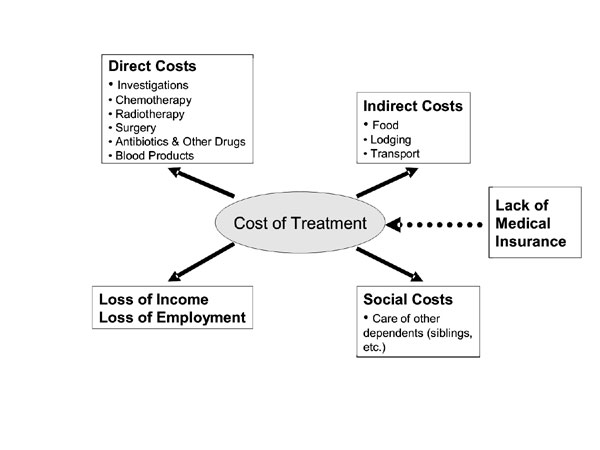 |
|
Fig. 1 The true "cost" of therapy in
childhood cancer. |
Other factors - Abandonment rates are suggested to
be related to lower educational status of parents but it may actually be a
confounder for financial status(5). In a multivariate analysis,
educational status of mother was not significant once monthly income had
been taken into account(10).
What are the Solutions?
There are models of successful interventions in certain
parts of the developing world. Most notable among them have been the
"twinning programs" between St Jude Children's Research Hospital in
Memphis, USA and multiple countries in the developing world, and links
established by Monza's International School of Pediatric
Hematology/Oncology and 14 Latin American countries(2,22). Twinning
fosters interaction between public hospitals in developing countries and
established cancer treatment centers in the developed world, with the goal
of improving survival rates among children with cancer.
As financial burden is the major cause of abandonment,
it would seem an obvious target for intervention. However, giving money to
patients for transport led to only a marginal improvement in abandonment
rates in Bolivia(23) and providing free chemotherapy did not prevent
abandonment in Indonesia(24). As Fig 1 shows, the true cost
of treatment is a complex interplay of multiple factors and interventions
are likely to work when they take all factors into account. Only when
poor, uneducated parents in Indonesia were given free chemotherapy and
equipped with the knowledge of how to access this resource, did
abandonment rates decrease(25). A multi-pronged approach of educating
healthcare providers to facilitate early diagnosis, developing a treatment
protocol, training ophthalmologists and donating essential equipment has
led to similar success in reduction of treatment refusal and abandonment
in those children treated with retinoblastoma in Central America(26).
Another area of focus to reduce abandonment has been in
the adaptation of established treatment protocols used in the developed
world to suit local needs. In the absence of robust supportive care,
giving intensely myelosuppressive therapy can lead to more harm than
benefit. This treatment-related toxicity is a major reason for abandonment
during the initial intensive phases of treatment. When children in Malawi
with Burkitt lymphoma were treated with a modified version of the French
LMB 89 protocol, treatment related deaths and abandonment rates were
high(27). Subsequently, the protocol was made short and less intense
making it cheaper and leading to shorter hospitalization. This
considerably reduced abandonment and decreased the number of
treatment-related deaths, albeit with a higher relapse rate(28). It is
important that treatment protocols are not only evidence-based but also
locally appropriate. In recognition of this, treatment strategies of
graduated intensity for ALL have been proposed for India as well as the
rest of the developing world(29,30). These take into account availability
of diagnostic, therapeutic and supportive care facilities as well as the
financial resources.
The Way Ahead
As clinicians providing care to children with cancer in
India, our role should not be limited to providing diagnostic and
therapeutic care. The onus is upon us to find strategies to deal with
those who refuse or abandon treatment. First, we need to get a more
accurate assessment of the size of the problem. Currently, hospital-based
cancer registries using web-based technology (e.g. www.pond4kids.org
internationally, and www.indiapod.org in the Indian context) are
prospectively collecting data on children with cancer including
information on refusal and abandonment rates. Centres in India providing
care to children with cancer should consider utilizing these freely
available resources.
Data collected from these registries can then be
analyzed for the relation of treatment refusal and abandonment to various
demographic, biologic, treatment-related and socio-economic variables.
This can be used to identify factors common to other developing nations as
well as those unique for India. This information will be useful to
generate hypotheses and plan interventions.
Interventions which have shown to effectively reduce
refusal and abandonment should be adopted by centres treating children
with cancer. Some of these include twinning with tertiary centres within
or outside the country; adapting established treatment protocols to local
needs; providing adequate pain relief during diagnostic and therapeutic
procedures; delivering clear and honest communication in a sensitive and a
culturally appropriate manner; and providing assistance with direct
medical costs as well as indirect ones like food, lodging and transport.
Conclusions
Refusal and abandonment is the leading cause of
treatment failure in children with cancer in the developing world.
Hitherto, there has been no systematic attempt to understand and address
this problem in India, and is urgently needed. There is a growing body of
research from other parts of the developing world which should serve as
useful pointers.
Contributors: RSA conceived the idea, searched the
literature and wrote the initial draft. He will act as guarantor of the
paper. TE and BP assisted in analysis and interpretation of the data and
critically revised the draft. All authors gave their approval to the final
version.
Funding: RS is funded by a grant from the
Paediatric Endowment Fund Christie Hospital NHS Foundation Trust and from
the Teenage Cancer Trust. TE holds a program grant from the Teenage Cancer
Trust.
Competing interests: None stated.
References
1. Stiller C, ed. Childhood Cancer in Britain:
Incidence Survival, Mortality. Oxford: Oxford University Press; 2007.
2. Howard SC, Pedrosa M, Lins M, Pedrosa A, Pui CH,
Ribeiro RC, et al. Establishment of a pediatric oncology program
and outcomes of childhood acute lymphoblastic leukemia in a resource-poor
area. JAMA 2004; 291: 2471-2475.
3. Kellie SJ, Howard SC. Global child health
priorities: what role for paediatric oncologists? Eur J Cancer 2008; 44:
2388-2396.
4. Metzger ML, Howard SC, Fu LC, Peńa A, Stefan R,
Hancock ML, et al. Outcome of childhood acute lymphoblastic
leukaemia in resource-poor countries. Lancet 2003; 362: 706-708.
5. Mostert S, Sitaresmi MN, Gundy CM, Sutaryo, Veerman
AJ. Influence of socioeconomic status on childhood acute lymphoblastic
leukemia treatment in Indonesia. Pediatrics 2006; 118: e1600-1606.
6. Kulkarni K, Marwaha RK, Trehan A, Bansal D. Survival
outcome in childhood ALL: experience from a tertiary care centre in North
India. Pediatr Blood Cancer 2009; 53: 168-173.
7. Arora RS, Eden T, Pizer B. The problem of treatment
abandonment in children from developing countries with cancer. Pediatr
Blood Cancer 2007; 49: 941-946.
8. Arora RS, Eden T, Kapoor G. Epidemiology of
childhood cancer in India. Indian J Cancer 2009; 46: 264-273.
9. Arora B, Kurkure P, Parikh P. Childhood cancers:
perspectives in India. J Indian Med Assoc 2005; 103: 479-842.
10. Bonilla M, Rossell N, Salaverria C, Gupta S, Barr
R, Sala A, et al. Prevalence and predictors of abandonment of
therapy among children with cancer in El Salvador. Int J Cancer 2009; 125:
2144-2146.
11. Sitaresmi MN, Mostert S, Schook R, Sutaryo, Veerman
AJ. Treatment refusal and abandonment in childhood acute lymphoblastic
leukemia in Indonesia: an analysis of causes and consequences.
Psycho-oncology 2010; 19: 361-367.
12. Yadav S, Anjan M, Sachdeva A. Outcome of children
with haematological malignancies who are lost to follow up [abstract]?
Pediatr Blood Cancer 2007; 49: 527.
13. Borooah VK. Gender bias among children in India in
their diet and immunisation against disease. Soc Sci Med 2004; 58:
1719-1731.
14. Pearce MS, Parker L. Childhood cancer registrations
in the developing world: still more boys than girls. Int J Cancer 2001;
91: 402-406.
15. Abuidris DO, Elimam ME, Nugud FM, Elgaili EM, Ahmed
ME, Arora RS. Wilms tumour in Sudan. Pediatr Blood Cancer 2008; 50:
1135-1137.
16. Yeh CH, Lin CF, Tsai JL, Lai YM, Ku HC.
Determinants of parental decisions on 'drop out' from cancer treatment for
childhood cancer patients. J Adv Nurs 1999; 30: 193-199.
17. Sachdeva A, Jain V, Yadav SP, Gupta S, Pruthi PK,
Arya SC. Move to alternative medicine why? and when? the Indian scenario
[abstract]. Pediatr Blood Cancer 2005; 45: 578.
18. Israëls T, Chirambo C, Caron H, de Kraker J,
Molyneux E, Reis R. The guardians' perspective on paediatric cancer
treatment in Malawi and factors affecting adherence. Pediatr Blood Cancer
2008; 51: 639-642.
19. Mostert S, Sitaresmi MN, Gundy CM, Sutaryo, Veerman
AJ. Parental experiences of childhood leukemia treatment in indonesia. J
Pediatr Hematol Oncol 2008; 30: 738-743.
20. Mostert S, Sitaresmi MN, Gundy CM, Sutaryo, Veerman
AJ. Attitude of health-care providers toward childhood leukemia patients
with different socio-economic status. Pediatr Blood Cancer 2008; 50:
1001-1005.
21. Chandy M. An approach to the management of leukemia
in the developing world. Clin Lab Haematol 2006; 28: 147-153.
22. Masera G, Baez F, Biondi A, Cavalli F, Conter V,
Flores A, et al. North-South twinning in paediatric haemato-oncology:
the La Mascota programme, Nicaragua. Lancet 1998; 352: 1923-1926.
23. Yolanda E, Estela C, Yolanda R, Cesar KJ.
Diminishing the abandonment of very poor children through giving them
money for transportation and strict controls from health nets, this
corresponds to My Child Matters project [abstract]. 8th INCTR Meeting on
Cancer in Countries with Limited resources; 2009 Mar 22-24; Antalya,
Turkey.
24. Mostert S, Sitaresmi MN, Gundy CM, Sutaryo, Veerman
AJ. Does aid reach the poor? Experiences of a childhood leukaemia
outreach-programme. Eur J Cancer 2009; 45: 414-419.
25. Mostert S, Sitaresmi MN, Gundy C, Janes V, Sutaryo
S, Veerman AJ. Comparing childhood leukemia treatment before and after
introduction of parental-education program in Indonesia. Arch Dis Child
2010; 95: 20-25.
26. Wilimas JA, Wilson MW, Haik BG, Barnoya M, Fu L,
Castellanos M, et al. Development of retinoblastoma programs in Central
America. Pediatr Blood Cancer 2009; 53: 42-46.
27. Hesseling PB, Broadhead R, Molyneux E, Borgstein E,
Schneider JW, Louw M, et al. Malawi pilot study of Burkitt lymphoma
treatment. Med Pediatr Oncol 2003; 41: 532-540.
28. Hesseling P, Broadhead R, Mansvelt E, Louw M,
Wessels G, Borgstein E, et al. The 2000 Burkitt lymphoma trial in
Malawi. Pediatr Blood Cancer 2005; 44: 245-250.
29. Chandy M. Childhood acute lymphoblastic leukemia in
India: an approach to management in a three-tier society. Med Pediatr
Oncol 1995; 25: 197-203.
30. Hunger SP, Sung L, Howard SC. Treatment strategies
and regimens of graduated intensity for childhood acute lymphoblastic
leukemia in low-income countries: A proposal. Pediatr Blood Cancer 2009;
52: 559-565.
|

