Sudha Chaudhari, Pradeep Suryawanshi, Shrikant
Ambardekar*, Manoj Chinchwadkar* and Arun Kinare*
From the Departments of Pediatrics and * Radiology,
King Edward Memorial Hospital, Pune 411 011, India.
Correspondence to Dr. Sudha Chaudhari, Consultant,
Division of Neonatology, Department of Pediatrics, King Edward
Memorial Hospital, Pune 411 011, India. E-mail:
kemhrc@vsnl.com
Manuscript received: February 2, 2004, Initial
review completed: March 22, 2004;
Revision accepted: June 15, 2004.
We conducted a case matched control study to
observe the adverse effects of ciprofloxacin used in neonatal
septicemia We enrolled 30 neonates with multidrug-resistant
septicemia who were treated with intravenous ciprofloxacin for
14 days. Thirty matched neonates with septicemia treated with
other antibiotics were enrolled as controls There was no
difference in the mean serum electrolytes, hepatic, renal and
hematologic parameters of the two groups. Serial
ultrasonographic measurements of the cartilage of the knee after
1 and 6 months showed no difference in the two groups. The
femoral cartilage showed an increase of 78.8% in the mean
longitudinal area after 6 months in the study group. In the
control group, the femoral cartilage showed a 78.4% increase
after 6 months. Similarly, the tibial cartilage showed no
difference in the percentage increase in size of the study and
control group at the end of 6 months. When controlled for birth
weight and gestation, cartilage size was not affected by
ciprofloxacin.
Key words: Arthropathy, Ciprofloxacin, Newborn.
Ciprofloxacin is an antibacterial agent with a
broad spectrum of activity, effective against various pathogens
like Pseudomonas aeruginosa and Klebsiella
pneumoniae. Notwithstanding its immense potential as a "life
saving" drug, its use is restricted in neonates and children due
to its adverse effects, especially on the joints and growing
cartilage(1).
Septicemia is one of the commonest causes of
mortality in the Neonatal Intensive Care Units (NICU) in India(2)
and multidrug resistant Klebsiella pneumoniae is the
commonest offending bacterial agent. Ciprofloxacin was used by us
in the treatment of multi-drug resistant septicemia on a
compassionate basis as a "life saving" drug. The main aim of this
prospective study was to observe the adverse effects of
ciprofloxacin - hepatic, renal, hematological and ocular
dysfunction, and especially its arthropatho-genic effect by doing
serial measurements of the cartilage using ultrasonography.
Subjects and Methods
This study was conducted in the Neonatal
Intensive Care Unit and "High Risk" follow up Clinic of Department
of Pediatrics, King Edward Memorial Hospital, Pune in 1999-2000.
Thirty neonates with septicemia who were already on other
antibiotics, but had shown clinical deterioration and the blood
culture had grown multi-drug resistant organisms sensitive to
ciprofloxacin were selected for the study. Ciprofloxacin was given
in a dose of 20 mg/kg in two divided doses as a slow intravenous
infusion over a period of 30 minutes, for a period of 14 days.
Informed consent of the parents was taken before starting this
treatment. Clearance from the ethical committee of the hospital
for use of ciprofloxacin was obtained before starting this study.
Thirty neonates with septicemia matched for
gender, gestational age, birth weight, and risk factors who were
being treated with other antibiotics (cefotaxime, amikacin),
formed the control group. The infants on ciprofloxacin were
scrutinized every day for objective evidence of arthropathy in the
form of swelling or tenderness over the joint, decreased
spontaneous movements or restriction and painful movements of the
joints.
Serum electrolytes, blood urea nitrogen, serum
creatinine, serum bilirubin, SGPT and alkaline phosphatase were
evaluated at the beginning and end of the treatment. Similarly, a
hemogram with peripheral blood smear and platelet count,
prothrombin time and partial thromboplastin time was done at the
beginning and end of the treatment.
Ultrasonography evaluation of the right knee
joint was done at the end of the treatment (day 15) by a trained
Ultrasonologist who was blinded to the group allocation, using
Ultramark 9 machine (Phillips, Bothell) with high frequency
broadband linear transducer. The following observations were made
on examination of the right knee joint (i) anteroposterior,
cranicaudal and transverse diameter and longitudinal and
transverse area of the lower end of femur and upper end of tibia;
(ii) "two layer" appearance of the cartilage which
separates the epiphyseal and articular cartilage, the articular
cartilage being less echogenic; (iii) fluid in the
suprapatellar bursa; and (iv) contour of the epiphyses.
These observations were recorded at the end of ciprofloxacin
therapy, and 1 month and 6 months after completion of therapy.
Anthropometry was recorded at birth and at the
6 month visit. An X-ray of the right knee joint,
anteroposterior and lateral views, was also taken at 6 months. An
ophthalmic check up was done to look for lenticular opacities and
cataract.
The means were compared using the unpaired ‘t’
test. A multiple linear regression analysis was done using
cartilage size as the outcome variable and birthweight,
gestational age and ciprofloxacin as co-variates.
Results
Thirty neonates treated for septicemia with
ciprofloxacin were followed up along with 30 neonates with
septicemia treated with other antibiotics. Both the study and
control group had 24 preterm and 6 full term neonates with 17
males and 13 females each. The birth weight in the ciprofloxacin
group ranged between 900-3400 g with a mean of 1688 ± 122 g,
whereas the mean birthweight was 1699 ± 105 g in the control group
(range 980-3360 g). The mean gestational age of the ciprofloxacin
group was 33.2 ± 3.83 weeks and that of the control group was 33.6
± 3.43 weeks (range 28 to 40 weeks).
The organisms isolated from the blood culture
of the ciprofloxacin group were Klebsiella pneumoniae in 20
(66.6%), Pseudomonas aeruoginosa in 5 (16.6%), coagulase
negative Staphylococci in 2 (6.6%) and gamma hemolytic
Streptococci in 1 (3.3%) neonate. The isolates were more
diverse in the control group with Klebsiella in 10 (33%),
Pseudomonas in 5 (16.6%), coagulase negative
staphylococci in 4 (13.3%), gamma hemolytic streptococcus
and enterococci in 3 (10%) each and Streptococcus
pneumoniae and Salmonella worthington in 1 case
each.
Treatment with ciprofloxacin was started
between 5th to 13th day of life with a mean of 8.06 days. Except
for serum bilirubin, all the biochemical and hematological
parameters were comparable between the two groups at the end of
therapy (Table I). Daily examination of the joints showed
no swelling, tenderness or restricted movements. Table II
shows that there was no significant difference between the
measurements of the lower femoral cartilages in the ciprofloxacin
and control group. Percentage increase in mean longitudinal area
of the lower femoral and upper tibial cartilage over 6 months was
similar in the two groups (Fig. 1). Ultra-sonography of the
right knee showed no effusion, a normal "two layer" appearance at
all the three examinations. There was no difference between the
cartilage size and growth of the cartilage in male and female
infants. A multiple linear regression analysis showed that
ciprofloxacin did not affect cartilage size, when controlled for
birth weight and gestation. X-ray of the knee joint (anteroposterior
and lateral view) taken at 6 months, showed no abnormality in any
of the infants.
TABLE I
Comparison of Biochemical and Hematologic Parameters of
Study and Control Group.
TABLE II
Measurements of the Cartilage of
Lower End of Femur.
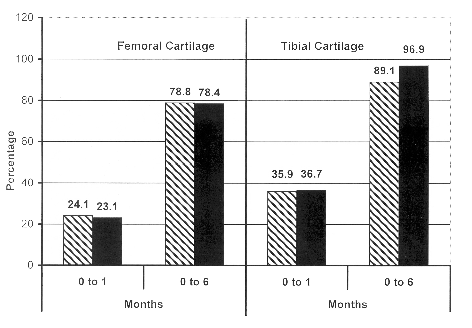 |
 |
|
Fig. 1. Percentage
increase in longitudinal area (mean) of femoral and tibial
cartilage. |
The length (height) velocity was calculated for
the first 6 months of life. This was 2.57 cm (SD = 0.53) in the
study group compared to 2.36 cm (SD = 0.35) in the control group
(p > 0.05). An ophthalmic check up revealed no lenticular
opacities or cataracts.
Discussion
Ciprofloxacin is used for a variety of
infections in adults. It has a broad anti-microbial spectrum,
marked bactericidal activity and favorable pharmacokinetics,
features which have made it a popular antibiotic in adult
practice. However, like other quinolones, it has not been approved
for use in children, primarily because of its potentiality to
cause irreversible damage to growing cartilage in weight bearing
joints in immature animals(3,4).
Ciprofloxacin was used on a compas-sionate
basis in children. Chysky, et al. used it in 634
adolescents and found it to be safe(5). They concluded that the
safety profile in children was no different from that in adults.
Bethell, et al.(6) used it in Vietnam to treat 137 children
with Salmonella infections and found no joint symptoms and
no alteration of growth. Martell, et al.(7) treated 7
preterm infants with sepsis and found no evidence of joint
involvement or growth failure. Pradhan, et al.(8) did
measurements of linear growth in addition to MRI scans in children
and found no cartilage toxicity. Gurpinar, et al.(9)
followed up 9 neonates upto the age of 2.5 years and found normal
growth and no osteoarticular problems.
Serial measurements of the cartilage by
ultrasonography did not show any adverse effects of ciprofloxacin
on the growing cartilage or any involvement of the joints in our
study. No other untoward adverse effects were detected in this
small series of neonates treated with ciprofloxacin. The main
limitations of this study are the small sample size and the short
follow up. However, since maximum growth of the cartilage and
epiphysis occurs during the first six months, we feel that no
arthropathogenic effects would manifest after this age. Hence
ciprofloxacin may be used with discretion to treat multi-drug
resistant septicemia in neonates, as a "life saving drug".
Contributors: SC conceived the project.
selected the cases and matched controls and supervised the
project, wrote the manuscript. PS collected the data. SA and MC
did ultrasonography. AK supervised ultrasonography. SC will act as
guarantor for the paper.
Funding: None.
Competing interests: None.
