H
eadache is common in
children and
adolescents. About 60% of children
worldwide report at least 3 attacks of
headache per year [1]. Tension-type headache (TTH) and migraine
are the most common headache disorders in schoolchildren with a
prevalence of 8 and 23%, respectively [2,3]. Despite the high
prevalence of headache in children, it continues to be
under-diagnosed and undertreated. Many children are managed at
home with over-the-counter medications, some children are
managed at primary care and only a small proportion of children,
with difficult to treat headache disorders, are referred and
managed at specialist pediatric services.
Migraine and, to a lesser degree, TTH are
well-studied and described in the pediatric literature, but
little is written on many others, especially rare primary
headache disorders and the uncommon variants and complications
of common headache disorders in children and adolescents. Except
for migraine, the three editions of the International
Classification of Headache Disorders (ICHD-1, ICHD-2 and
ICHD-3), provided definitions and criteria for the diagnosis of
all headache disorders derived from studies on and experience in
adult patients [4-6]. It is conceivable that the clinical
presentations of most, if not all, primary headache disorders in
children can be different than those in adults [7]. The
differences can be due to the inherent nature of the disease
itself, neurodevelopmental factors, biopsychosocial influences
of the disease, life-style and education, and also children’s
response to pharmacologic and non-pharmacologic therapies.
Therefore, studies on uncommon headache disorders in children
and adolescents are badly needed in order to better define the
conditions and inform management decisions.
CLASSIFICATION
Headache disorders are classified on the
basis of etiology into primary (no other underlying cause),
secondary (when headache is a manifestation of another disorder)
and undetermined etiology (Box I). Headaches are also
sub-classified on the basis of frequency of attacks and duration
of the headache disorder into episodic (less than 15 days per
month) or chronic (attacks occur on at least 15 days per month
over at least three consecutive months). Migraine is further
sub-classified according to clinical features (different types
of migraine) and trigeminal autonomic cephalalgias (TACs) are
sub-classified on the basis of attack duration.
|
Box I Classification of Most Common
Headache Disorders in Children
Primary headaches
• Migraine
• Tension-type headache
• Trigeminal autonomic cephalalgias
• Others
Secondary headache
• Medication overuse headache
• Posttraumatic headache
• Brain tumors
• Idiopathic intracranial
hypertension
Others
• Cranial neuropathies
• Facial neuropathies
• Others
|
ASSESSMENT OF THE CHILD WITH HEADACHE
In the absence of diagnostic tests and
biomarkers, the diagnoses of primary headache disorders are
based on the clinical features and globally acceptable
definitions and diagnostic criteria. A focused and detailed
clinical history is essential in assessment of children with
headache in order to make a positive diagnosis, and appropriate
classification. Making the right diagnosis allows explaining the
condition to the child and the family, making a rational
decision on investigations if needed, offering the most
appropriate treatment options and helps in predicting prognosis.
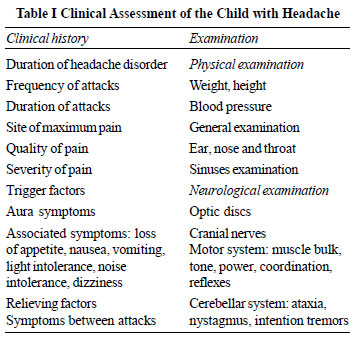
Essential elements of clinical history, general examination and
neurological exami-nation are summarized in Table I and
management workup may follow the steps as shown in Fig. 1.
 |
| |
| |
| |
 |
The severity of pain is best assessed by its
effects on behavior and activities; severe headache stops all
activities during attacks, moderate headache stops some but not
all activities, and mild headache dose not interfere with normal
daily activities. Absence of symptoms between attacks and
complete return to normal self is an important feature of
primary headache. Secondary headaches should be suspected and
considered if red flags are detected on the clinical history,
and physical and neurological examinations (Box II).
|
Box II Red Flags and Indications for
Investigations
Clinical history
Side locked headache
Acute progressive headache
Vomiting on waking up
Deteriorating vision
Seizures
Personality change
Persisting symptoms between attacks
Physical examination
Hypertension
Faltering growth
Delayed puberty
Neurological examination
Papilledema
New neurological deficit
Ataxia
Nystagmus
New squint
|
The diagnosis of common primary headache
disorders such as migraine and tension-type headache can be made
confidently on clinical history, normal examination, absent red
flags and on the application of the ICHD-3 criteria. Other less
common headache disorders may cause difficulties in diagnosis
and management and will be the subject for this review.
GENERAL MANAGEMENT STRATEGIES
Exploring and addressing the concerns of
patients and their families are the first important steps in
successful management. Advice on healthy life style – regular
meals, sleep, exercise and rest may reduce impact and improve
coping with headache.
For acute treatment, simple analgesics should
be used in appropriate dosages and as early as possible after
onset of headache. Children should avoid taking painkillers on
more than three days per week in order to avoid medication
overuse headache (MOH). Triptans can also be given in acute
migraine attacks in accordance with local license and
regulations and with similar precautions to avoid MOH.
Evidence-based recommendation in the
prevention of migraine can be hard to find with conflicting
evidence, however treatment with propranolol, topiramate,
flunarizine and amitriptyline can be considered on individual
basis. Specific treatment for rare headache disorders will be
discussed separately.
CHRONIC MIGRAINE
Migraine is a common disorder with a
prevalence of around 10% in schoolchildren [1], and chronic
migraine (CM) is a subtype of migraine. The diagnosis of CM is
made when headache occurs on at least 15 days per month on at
least 3 consecutive months, of which at least on 8 days per
month the headaches are those of migraine [6].
Migraine may present as CM from the onset,
but it can also evolve over a period of time in children with
episodic migraine. It is estimated that about 2% of adolescents
suffer from CM and at least half the patients overuse
medication; simple analgesics or anti-migraine drugs such as
sumatriptan [8]. CM is more common in girls than boys and more
prevalent in adolescents than in younger children. CM has a
significant impact on the child’s quality of life, school
attendance and educational attainment as compared to children
with episodic migraine and control healthy children [8].
Management
The management of children with CM can be
difficult and, ideally, needs a multidisciplinary approach and a
positive contribution from parents and guardians, clinical
psycho-logy services, education and school teachers and also
from school nurses. Investigations are not necessary except in
presence of red flags or new abnormalities on neurological
examination. An individual migraine management plan agreed upon
by the child, the parents and members of the multi-disciplinary
team (MDT) promotes better manage-ment of headache at home and
at school and may reduce impact on education and quality of
life.
Medical management starts with reducing the
risk of medication overuse and treatment of MOH when present.
Pain killers should, therefore be avoided as they have a limited
role. Preventive drugs aim to reduce the frequency and the
severity of migraine attacks and to improve the quality of life.
Several medications are used but with sometime, a conflicting
evidence for their effectiveness. Amitriptyline, with and
without cognitive behavioral therapy (CBT), topiramate,
flunarizine and botox are commonly offered to patients [9-11].
Greater occipital nerve block and botox injections are possible
future management options. Calcitonin-gene related peptide
(CGRP) monoclonal antibodies are shown to be successful in
migraine prevention in adults, but still awaiting trials and
licensing in children.
Prognosis: The prognosis and the
long-term course of CM have not been well studied in children,
but the impact on quality of life is a consistent feature and
studies showed reduced educational attainment and earning power
during adult life [12].
HEMIPLEGIC MIGRAINE
Hemiplegic migraine (HM) is a form of
migraine with motor aura and depending on presence or absence of
other affected family members; it is divided into familial
hemiplegic migraine (FHM) or sporadic hemiplegic migraine (SHM).
FHM is sub-classified according to the underlying genetic
mutation; FHM1 is associated with a mutation on the CACNA1A
gene, FHM2 on the ATP1A2 gene, FHM3 on SCN1A and
FHM (other loci) when no genetic mutation can be found despite
the familial occurrence of the disease.
The prevalence of HM is not known, but is
considered rare. About 2% of patient seen at a specialist
children headache clinic have HM [13]. Girls are more commonly
affected than boys and its peak incidence is during adolescence.
The clinical presentation of HM can be
distressing to the child, frightening to the family and can pose
a dilemma in diagnosis for clinicians. Attacks can be triggered
by minor head trauma and followed by complex aura; a combination
of visual, sensory, speech and motor symptoms. The headache that
follows can be severe and is often associated with intense
nausea and vomiting that may lead to confusion and dehydration.
A recent study on a cohort of 46 children
with HM showed that children present with fewer non-motor auras
than adults and the first attack may be preceded by transient
neurological signs and symptoms especially in early childhood
[14]. The attacks can last over 1-2 days and children will be,
invariably, investigated with neuroimaging to rule out space
occupying lesions, intracranial bleeding or arterial ischemic
strokes. Investigation will always be necessary, especially on
the first presentation as the diagnosis, as defined by (ICHD-3),
can only be made after at least two fully reversible attacks.
Distinguishing HM from stroke can be
difficult on clinical features alone as they share many
symptoms. Standard MRI of the brain may not be helpful and
specialist cerebral perfusion studies using functional MRI and
arterial spin labeling (ASL), only available at limited centers,
may demonstrate areas of cerebral hypoperfusion soon after onset
of symptoms and hyperperfusion 12-14 hours after onset. The
changes in perfusion are not limited to the territories of the
main cerebral arteries but they are evident across the
boundaries suggesting neural rather than vascular basis of this
phenomenon [15,16].
Management
Treatment of acute attacks once the diagnosis
is established should aim at reassuring the child and parents,
providing effective pain relief, hydration and monitoring of
symptoms. Simple analgesics (paracetamol 10-20 mg/kg or
ibuprofen 7.5-10 mg/kg) are the preferred options. The use of
triptans is currently not recommended as all clinical trials
excluded children and adults with HM and there is no evidence of
their safety in children and adolescents. The exclusion from
trials was based on the theoretical risk of triptans
exacerbating cerebral vasoconstriction and causing cerebral
infarction.
Care should be taken to prevent dehydration
by encouraging oral fluids, but intravenous fluids may be
necessary. Antiemetic medications such as metoclopra-mide or
ondansetron may also be needed.
Preventative treatment may be necessary if
attacks are prolonged, frequent or causes distress to child and
parents. Topiramate, amitriptyline and flunarizine in particular
are good treatment options.
Prognosis: Counseling of patients should
take into account the known natural history of the disease,
which is characterized by periods of remissions and relapses.
The attacks of HM tend to be frequent and severe during
adolescence and also in late adult life with periods of
remission in between. A follow up of eight family members for a
mean period of ten years showed the disease to be clinically
stable [17]. Children with FHM1 due to associated CACNA1A
gene mutation have an increased risk of progressive ataxia in
late adult life and children with FHM3 due to SCN1A
mutation are at a higher risk for epilepsy.
THUNDERCLAP HEADACHE
Thunderclap headache (TH) is a term given to
describe sudden severe headache that reaches its peak intensity
within seconds or minutes and persists for hours. TH may become
recurrent over several weeks if untreated. TH is a secondary
headache in most cases and a diagnosis of primary TH should only
be made after full, appropriate and timely investigations
including neuroimaging. The prevalence of TH in children and
adolescents is not known and it is rare in pediatric clinical
practice. Cases reported in children are mostly secondary to
sinus venous thrombosis [18].
The clinical features of TH in children are
probably similar to those in adults. The clinical picture is
usually dominated by the abrupt onset of the headache and its
severe intensity described by patients as the most severe
headache they have ever experienced, prompting them to seek
urgent medical advice and assessment. The headache attacks can
be brief, but repetitive. Although the criteria for the
diagnosis of TH in ICHD-3 (Box III) are that of primary
TH, it is always necessary to exclude underlying intracranial
vascular disease.
|
Box III Criteria for Diagnosis
of Thunderclap Headache
A. Severe head pain fulfilling
criteria B and C
B. Abrupt onset reaching maximum
intensity in <1 min
C. Lasting for
³5
min
D. Not better accounted for by another ICHD-3
diagnosis.
|
| |
Secondary TH: TH may be the
presenting symptoms in patients with several intracranial
vascular disorders. The most common causes of TH in children and
adolescents are intracerebral haemorrhage, cerebral venous
thrombosis, subarachnoid haemorrhage with or without ruptured
arterial aneurysms and reversible cerebral vasoconstriction
syndrome (RCVS). Secondary TH may not be
immediately distinguishable from primary TH and therefore full
neurological examination and measurement of arterial blood
pressure are mandatory. Prompt recognition and urgent
appropriate assessment start at emergency department in order to
avoid life-threatening complications [19].
Management
Investigations at presentation should include
brain MRI and MR angiography. Other investigations should also
include CSF opening pressure, CSF microscopy, culture, protein
and glucose (paired with blood glucose) and examination for
xanthchromia at appropriate time interval from presentation. MRA
may need to be repeated if RCVS is highly suspected, as
vasoconstriction may not be apparent in the early stages of the
disease.
Treatment aims to relieve symptom while
addressing the management requirements of the underlying
condition.
CHIARI MALFORMATIONS HEADACHE
Chiari malformation is a congenital anomaly
of the posterior cranial fossa characterized by crowding of its
contents, a caudal displacement of the cerebellar tonsils and
brainstem through the foramen magnum and an associated expansion
of the CSF spaces in the cervical and possibly the thoracic
spinal canal creating a static or progressive syrinx. Chiari
malformation is classified into types 1-4 depending on the
degree of malformation, the extent of cerebellar and brainstem
herniation into the foramen magnum, cerebellar hypoplasia,
syringomyelia, obstruction of CSF flow and presence of an
encephalocele.
Chiari malformation type 1 (CM1) is the most
common form and it is asymptomatic in the vast majority of
cases. In CM1, there is mild to moderate crowding of the
posterior fossa and a descent of cerebellar tonsils between 5-10
mm below the foramen magnum with no or a very small syrinx. It
is commonly reported as an incidental finding in about 1% of
children and young people undergoing brain and spine MRI [20].
Headache due to CM1 is described in the
ICHD-3 as brief episodes lasting less than 5 minutes of
occipital or suboccipital pain, precipitated by cough or
Valsalva maneuver and it remits after successful treatment of
CM1 [6]. It is important to keep in mind that children with CM1
may also complain of other types of headache such as migraine
and tension-type headache, and they should not be confused with
CM1 headache. On rare occasions, children with CM1 may present
with other symptoms related to brainstem or cerebellar
dysfunction including visual disturbances, dysphonia, dysphagia,
sleep apnea, incoordination and sensory disturbances.
The diagnosis of CM1 is usually made on the
sagittal MRI of the brain and the cervical spine. The management
of children with CM1 is non-surgical in most patients after
discussion with a neurosurgeon and a neuroradiologist. Medical
management should include appropriate manage- ment of the pain
symptoms and a follow up imaging over a period of time to
confirm the non-progressive nature of the malformation and the
size of the syrinx, if present, in particular [21].
Surgery is only recommended for patients with
features suggesting brainstem or cerebellar compression, a large
or progressive syringomyelia or with a poorly controlled,
typical CM1 headache disorder. A single center experience in the
surgical treatment of children with CM1 over 25 years showed
that children with typical CM1 headache who were treated with
foramen magnum decompression (FMD) plus duraplasty achieved a
greater improvement in their headache than those treated with
FMD alone [22].
PRIMARY STABBING HEADACHE
Primary stabbing headache (PSH) is an
uncommon syndrome, also called ice-pick headache, characterized
by very short attacks. While ICHD-2 states that pain is confined
exclusively to the trigeminal territory, it has been accepted in
ICHD-3 to cross beyond the boundaries of the trigeminal nerve
and can be unilateral or bilateral in location (Box IV).
The typical stab lasts a few seconds; however, attacks lasting
up to 15 minutes in children and adolescents have been reported
[23].
|
Box IV Criteria for the Diagnosis of
Primary Stabbing Headache
A. Head pain occurring spontaneously
as a single stab or series of stabs and fulfilling
criteria B and C
B. Each stab lasts for up to a few
seconds
C. Stabs recur with irregular
frequency, from one to many per day
D. No cranial autonomic symptoms
E. Not better accounted for by
another ICHD-3 diagnosis.
|
Although the exact prevalence of PHS in the
pediatric population is unknown, 4-5% of children referred to
headache centers have PSH [23-25]. In an Italian study, 12.4% of
children with headache younger than 6 years had PSH [26]. Girls
and boys are equally affected, though in adults PSH is more
common in females [27]. Other primary headaches; migraine and
TTH may coexist with PSH in children [28-30]. Associated
symptoms, such as photo-phobia, phonophobia, nausea, and
dizziness, have been described in 20-50% of patients. Absence of
autonomic symptoms is the main feature that differentiates PSH
from trigeminal autonomic cephalalgias.
The pathophysiology of PSH is unknown, but
spontaneous firing of trigeminal fibers or abnormalities of the
descending pain control have been suggested as possible
mechanisms [27]. The relatively higher prevalence of PSH in
younger children suggests that PHS may be a precursor of
migraine/TTH [16].
Treatment of PSH in children is often not
necessary, unless the stabs are very frequent and interfere with
normal activities. Evidence for any medications in the treatment
of PSH is lacking, but indomethacin may offer an excellent
relief of pain when given in a dose of 25 mg three times per day
for 6-8 weeks alongside omeprazole for gastric protection.
Melatonin, amitriptyline, propanol, and COX2-inhibitors were
effective in adults and may be used in children [27].
TRIGEMINAL AUTONOMIC CEPHALALGIAS
Trigeminal Autonomic Cephalalgias (TACs) are
also uncommon, especially in pediatric age. However, they must
be considered even in young children, in order to offer the
appropriate treatment. They include cluster headache (CH),
Paroxysmal hemicrania (PH), Hemicrania Continua (HC), and
Short-lasting Unilateral Neuralgiform headache attacks with
Conjunctival injection and Tearing (SUNCT) or with cranial
autonomic symptoms (SUNA). Table II presents the
characteristics and the differential diagnosis of these
conditions.
Cluster Headache
The prevalence of cluster headache (CH) in
the pediatric population is around 0.1% [31]. CH is
characterized by severe and sometimes excruciating unilateral
pain, mainly in the orbital, supraorbital, and/or temporal
region, lasting 15-180 minutes and recurring up to 8 attacks per
day. Attacks are often associated with restlessness and/or
ipsilateral autonomic symptoms, such as conjunctival injection,
lacrimation, nasal congestion, rhinorrhea, eyelid edema,
forehead and facial sweating, miosis, and ptosis. The usual
presentation of CH is that of recurrent bouts of headache, each
bout consisting of several distinct headache attacks. In
episodic CH, the bouts of headache last from 7 days to 1 year,
and are separated by headache-free periods without treatment of
at least 3 months. In chronic CH, the headache-free period
between bouts is shorter than 3 months. It has been recently
suggested that the attacks can be shorter and less frequent in
children than in adults and restlessness can be more difficult
to demonstrate [7,32].
CH is more common in children over 10 years
of age, but it has been reported in children younger than 6
years of age [33,34]. Complex genetic factors are probably
involved in CH etiology [35,36]. However, it was noted that
there is a low prevalence (9%) of CH in the relatives of young
patients [34]. Environmental factors such as high exposure to
second hand smoking has been suggested as a risk factor for CH
development [34,37].
Treatment: Treatment of acute attacks in
children and adolescents consists of administering, as early as
possible after onset, either sumatriptan 10 mg or zolmitriptan 5
mg (as nasal spray), which are the only licensed triptans for
adolescents 12-18 years of age in Europe as well as high flow
100% oxygen at 12-15 L/min with a rebreathing mask [38].
For the prevention of CH, verapamil is the
drug of first-choice in adults. It has been used at the dose of
3-10 mg/kg/d in children and adolescents [38]. However,
verapamil can be difficult to manage in pediatric age because of
its side effects (effect on length of the PR interval and the
negative inotropic effect). Alternative treatments are melatonin
(0.1-0.2 mg/kg/d) and topiramate (1-2 mg/kg/d). A short course
of steroids like prednisone 2 mg/kg/day is reported to stop the
cluster within 5 days [34].
Greater occipital nerve block was shown to be
effective in three children [39].
Paroxysmal Hemicrania
Paroxysmal hemicrania (PH) is characterized
by short lasting (2-30 min), multiple and unilateral pain
attacks, with a typical attack frequency of more than 5 per day.
Pain is commonly associated with ipsilateral cranial autonomic
symptoms. PH is defined as episodic when attacks last from 7
days to 1 year and are separated by time intervals longer than 3
months. In chronic PH, the attack has to last more than 1 year
without interruption or with pain-free intervals shorter than 3
months. In children, PH can be atypical with bilateral pain,
attack duration longer than 30 minutes and attack frequency less
than 5 per day making it difficult to differentiate from CH
[7,40,41].
PH responds well to treatment with
indomethacin, making it a good therapeutic first line option in
all patients with unilateral short-lasting pain, associated with
cranial autonomic symptoms.
Hemicrania Continua
Hemicrania continua (HC) is characterized by
continuous unilateral headache with exacerbations of moderate or
greater intensity for at least 3 consecutive months. As in PH,
cranial autonomic symptoms ipsilateral to pain and/or sense of
restlessness are needed for the diagnosis. The response to
indomethacin should be complete, but can be variable in some
children. Only a few pediatric cases of HC have been published
with one patient responding to treatment with Botulinum toxin A
[42,43].
SUNCT and SUNA
These short-lasting neuralgiform attacks,
lasting from 1 to 600 sec, involve the trigeminal territory
unilaterally. The attacks can be isolated or can recur in
series. Pain is associated with conjunctival injection and/or
tearing in SUNCT or other cranial autonomic symptoms, such as
nasal congestion and/or rhinorrhea, eyelid edema, forehead and
facial sweating, miosis, and/or ptosis in SUNA. Only four cases
of pediatric SUNCT (3 idiopathic and 1 symptomatic) have been
described thus far [7].
CONCLUSIONS
Pediatricians are familiar with the diagnosis
and treatment of common headache disorders in children and
adolescents. Awareness of the different types of atypical or
rare headache disorders allows better assessment and a more
successful management. Learning points related to these
disorders are detailed in Box V.
|
Box V Learning Points for
Uncommon Pediatric Headache Disorders
Chronic migraine
• Affects 1-2% of adolescents
• Management requires
multidisciplinary approach in most patients and
realistic targets
• Management aims to revert
chromic migraine to episodic migraine
• Emphasis on life style factors
and preventive treatment· Avoid medication overuse
and address it if present
Hemiplegic migraine
• Diagnosis of HM can only be
made after at least 2 fully reversible episodes
• Investigations and neuroimaging
will be necessary at first presentation
• Triptans are not recommended
for treatment of acute attacks
• Flunarizine may be a good
option for the prevention of HM
Thunderclap headache
• TH presents with sudden onset
severe headache that reaches its peak within minutes
• Always exclude underlying
intracranial cause by appropriate investigations
Chiari malformation 1
• CM1 can be asymptomatic
incidental finding in about 1% of people
• Headaches due to CM1 are short,
occipital and triggered by Valsalva maneuver.
Discuss with a neurologist, a neurosurgeon and a
pediatric neuro-radiologist
• Surgical treatment only
required in a small proportion of patients
Primary stabbing headache
• Headache due to PSH are very
brief and repetitive
• No associated autonomic
features
• Excellent response to
indomethacin can be expected
Trigeminal autonomic cephalalgias
• Duration of attacks are
important in diagnosis, but acknowledge some overlap
• At least one autonomic feature
is present
• Agitation and distress are
important features
• PH and HC respond to
indomethacin in many patients
• Nasal sumatriptan and high flow oxygen are
effective acute treatment for CH.
|
Diagnosis should be based on the recognized
clinical criteria of the ICHD. Investigations to exclude serious
underlying neurological disorders may be necessary, but should
be interpreted with caution, in order to avoid over-diagnosis of
incidental findings such as Chiari malformation 1. Chronic
migraine can be associated with adverse impact on quality of
life and can be difficult to manage. Hemiplegic migraine may
pose diagnostic difficulties making investigations necessary
including MR angiography, especially at first presentation.
Trigeminal autonomic cephalalgias and stabbing headache are
relatively rare in children, but with the correct use of
diagnostic criteria, management plans with appropriate treatment
options and realistic expectations, it is possible to address
the patient needs.
Contributors: All authors
contributed equally and approved the final manuscript.
Funding: None; Competing interest:
None stated.
REFERENCES
1. Abu-Arafeh I, Russell G. Prevalence of
headache and migraine in schoolchildren. Brit Med J.
1994;309:765-9.
2. Abu-Arafeh I, Razak S, Sivaraman B,
Graham C. The prevalence of headache and migraine in
children and adolescents: A systematic review of
population-based studies. Dev Med Child
Neurol. 2010;52:1088-97.
3. Laurell K, Larsson B, Eeg-Olofsson O.
Prevalence of headache in Swedish schoolchildren, with a
focus on tension-type headache. Cephalalgia, 2004;24:380-8.
4. Headache Classification Committee of
the International Headache Society. Classification and
Diagnostic Criteria for Headache Disorders, Cranial
Neuralgias and Facial Pain. Cephalalgia. 1988;8:1-96.
5. Headache Classification Committee of
the International Headache Society (IHS). The International
Classification of Headache Disorders, 2nd edition.
Cephalalgia. 2004;38:1-211.
6. Headache Classification Committee of
the International Headache Society (IHS). The International
Classification of Headache Disorders, 3rd edition.
Cephalalgia. 2018;24:1-152.
7. Ozge A, Faedda N, Abu-Arafeh I, et al.
Experts’ opinion about the primary headache diagnostic
criteria of the ICHD-3rd edition beta in children and
adolescents. J Headache Pain, 2017;18:11.
8. Lipton RB, Manack A, Ricci JA, et al.
Prevalence and burden of chronic migraine in adolescents:
Results of the chronic daily headache in adolescents study
(C-dAS), headache, 2011;51:693-706.
9. Powers SW, Coffey CS, Chamberlin LA,
et al. Trial of amitriptyline, topiramate, and placebo for
pediatric migraine. NEJM. 2017;376:115-24.
10. Power SW, Kashikar-Zuck SM, Allen JR,
et al. Cognitive behavioral therapy plus amitriptyline for
chronic migraine in children and adolescents: A Randomized
Clinical Trial. JAMA. 2013;310:2622-30.
11. Le K, Yu D, Wang J, et al. Is
topiramate effective for migraine prevention in patients
less than 18 years of age? A meta-analysis of randomised
controlled trials. J Headache Pain. 2017;18:4-10.
12. Adams AM, Serrano D, Buse DC, et al.
The impact of chronic migraine: The chronic migraine
epidemiology and outcomes (CaMEO) study methods and baseline
results. Cephalalgia. 2015;35:563-78.
13. Abu-Arafeh I, MacLeod S. Serious
neurological disorders in children with chronic headache.
Arch Dis Child. 2005;90:937-40.
14. Toldo I, Brunello F, Morao V, et al.
First attack and clinical presentation of hemiplegic
migraine in pediatric age: A multicenter retrospective study
and literature review. Front Neurol.
2019;10:1079.
15. Boulouis G, Shotar E, Dangouloff-Ros
V, et al. Magnetic resonance imaging arterial-spin-labeling
perfusion alterations in childhood migraine with atypical
aura: a case–control study. Dev Med Child Neurol.
2016;58:965-9.
16. Cadiot D, Longuet R, Bruneau B, et
al. Magnetic resonance imaging in children presenting
migraine with aura: Association of hypoperfusion detected by
arterial spin labelling and vasospasm on MR angiography
findings. Cephalalgia. 2018;38:949-58.
17. Indelicato E, Nachbauer W, Eigentler
A, et al. Ten years of follow-up in a large family with
familial hemiplegic migraine type 1: Clinical course and
implications for treatment. Cephalalgia. 2018;38:1167-76.
18. Gosh PS, Rothner AD, Zakha KG,
Friedman NR. Reversible cerebral vasoconstriction syndrome:
A rare entity in children presenting with thunderclap
headache. J Child Neurol. 2011; 26:1580-4.
19. Raucci U, Vecchia ND, Ossella C, et
al. Management of childhood headache in the emergency
department: review of the literature. Front Neurol.
2019;10:886.
20. Aitkin LA, Lindan CE, Sidney S, et
al. Chiari type I malformation in a pediatric population.
Pediatr Neurol, 2009;40:449-54
21. Abu-Arafeh I, Campbell E. Headache,
Chiari malformation type 1 and treatment options, Arch Dis
Child 2017;102:210-1.
22. Raza-Knight S, Mankad K, Prabhakar P,
et al. Headache outcomes in children undergoing foramen
magnum decompression for Chiari I malformation. Arch Dis
Child. 2017;102:238-43.
23. Fusco C, Pisani F, Faienza C.
Idiopathic stabbing headache: Clinical characteristics of
children and adolescents. Brain Dev. 2003;25:237-40.
24. Kramer U, Nevo Y, Neufeld MY, et al.
The value of EEG in children with chronic headaches. Brain
Dev, 1994;16:304-8.
25. Vieira JP, Salgueiro AB, Alfaro M.
Short-lasting headaches in children. Cephalalgia.
2006;26:1220-4.
26. Raieli V, Eliseo M, Pandolfi E, et
al. Recurrent and chronic headaches in children below 6
years of age. J Headache Pain. 2005;6:135-42.
27. Hagler S, Ballaban-Gil K, Robbins MS.
Primary stabbing headache in adults and pediatrics: A
review. Curr Pain Headache Rep. 2014;18:450
28. Soriani S, Battistella PA, Arnaldi C,
et al. Juvenile idiopathic stabbing headache. Headache.
1996;36:565-7.
29. Abu-Arafeh I. Primary stabbing
headache: The need for a definition for children and
adolescents. Dev Med Child Neurol. 2020;62:10.
30. Ahmed M, Canlas J, Mahenthiran M, Al-Ani
S. Primary stabbing headache in children and adolescents.
Dev Med Child Neurol. 2020;62:69-74.
31. Ekbom K, Ahlborg B, Schéle R.
Prevalence of migraine and cluster headache in Swedish men
of 18. Headache 1978;18:9-19.
32. McAbee GN. A review of episodic and
chronic pediatric headaches of brief duration. Pediatr
Neurol. 2015;52:137-42.
33. Majumdar A, Ahmed MA, Benton S.
Cluster headache in children - experience from a specialist
headache clinic. Eur J Paediatr Neurol. 2009;13:524-9.
34. Mariani R, Capuano A, Torriero R, et
al. Cluster headache in childhood: case series from a
pediatric headache center. J Child Neurol. 2014;29:62-5.
35. Schurks M. Genetics of cluster
headache. Curr Pain Headache Rep. 2010;14:132-9.
36. Sjostrand C, Russell MB, Ekbom K, et
al. Familial cluster headache: Demographic patterns in
affected and nonaffected. Headache. 2010;50:374-82.
37. Rozen TD. Childhood exposure to
second-hand tobacco smoke and the development of cluster
headache. Headache. 2005;45:393-4.
38. Mack KJ, Goadsby P. Trigeminal
autonomic cephalalgias in children and adolescents: Cluster
headache and related conditions. Semin Pediatr Neurol.
2016;23:23-6.
39. Puledda F, Goadsby PJ, Prabhakar P.
Treatment of disabling headache with greater occipital nerve
injections in a large population of childhood and adolescent
patients: A service evaluation. J Headache Pain. 2018;19:5.
40. Tarantino S, Vollono C, Capuano A, et
al. Chronic paroxysmal hemicrania in paediatric age: Report
of two cases. J Headache Pain. 2011;12:263-7.
41. Raieli V, Cicala V, Vanadia F.
Pediatric paroxysmal hemicrania: A case report and some
clinical considerations. Neurol Sci. 2015;36:2295-6.
42. Fragoso YD, Machado PC. Hemicrania
continua with onset at an early age. Headache.
1998;38:792-3.
43. Prasad M, Ramdas S, Abu-Arafeh I. A child with hemicrania
continua phenotype responsive to Botulinum toxin-A. J Pediatr
Neurol. 2012;10:155-9.

