|
|
|
Indian Pediatr 2019;56: 647-652 |
 |
Prediction of Severe Acute Kidney Injury
using Renal Angina Index in a Pediatric Intensive Care Unit
|
|
Jitendra Gawadia, Kirtisudha Mishra, Manish Kumar and
Diganta Saikia
From Department of Pediatrics, Chacha Nehru Bal
Chikitsalaya, Geeta Colony, Delhi, India.
Correspondence to: Dr Kirtisudha Mishra, Department
of Pediatrics, Chacha Nehru Bal Chikitsalaya, Geeta Colony, Delhi 110
031, India.
Email: [email protected]
Received: July 22, 2018;
Initial review: December 27, 2018;
Accepted: May 20, 2019.
|
|
Objectives: To determine the
proportion of children in a pediatric intensive care unit with a
positive Day 0 Renal angina index who develop severe acute kidney injury
(AKI) on Day 3; and to compare the predictive ability of the index with
that of individual markers of renal injury, for the development of
severe acute kidney injury. Design: Observational study.
Setting: Pediatric intensive care unit of a tertiary-care hospital.
Participants: Consecutive children, 1 month to 12 years, admitted
in Level 3 pediatric intensive care unit for a minimum of 8 hours,
having weight and intake-output records, were eligible. Children known
to have chronic kidney disease or already in stage 2/3 acute kidney
injury/dialysis were excluded. Procedure: Day 0 Renal angina
index was calculated from the product of Risk Group score
(Pediatric intensive care admission/Ventilation and inotropy) and Renal
Injury score (fluid overload over previous 8 hours or the % fall in
estimated creatinine clearance from baseline). Renal angina index
³8 was
considered positive. Main outcome measure: The proportion of
children with positive Day 0 Renal angina index who develop severe AKI
(Kidney Disease Improving Global Outcomes (KDIGO)
³ Stage
2) on Day 3. Results: Of 162 enrolled children (median (IQR) age
10.5 (3,39) months), 86 (53%) had positive Renal angina index. On Day 3,
a higher proportion of children with positive index developed severe
AKI, compared to negative group (RR 95.5; 95% CI 21.7,420.5; P<0.001).
Day 0 positive Renal angina index had a sensitivity, specificity,
positive predictive value and negative predictive value of 96.9%, 75.5%,
72% and 97.4% respectively, for predicting severe AKI on Day 3. The
Receiver Operating Characteristic curve of Day 0 renal angina scores
showed AUC of 0.90 (95% CI 0.85, 0.95), better than the AUC obtained
from either Day 0 serum creatinine or Day 0 percent fall in estimated
creatinine clearance from baseline. Conclusion: Day 0 Renal
angina index positivity is a promising tool to identify critically ill
children with impending severe AKI.
Keywords: Acute renal failure, Creatinine,
Management, Outcome.
|
|
T
here is a pressing need to identify subsets of
critically ill children who are at high risk for incurring severe renal
injury, which has been shown to have detrimental effects both in short
and long term [1-3]. The essential marker by which acute kidney injury
(AKI) has been diagnosed for years is serum creatinine, which
deceptively remains normal till much of the renal injury has already
occurred [4,5]. The diagnostic performance of biomarkers of AKI has been
poor when used in a heterogenous population [6,7].
To prevent delayed recognition of AKI, it is
imperative to have a composite set of clinical and laboratory
parameters, the combined presence of which is likely to predict severe
AKI. To this end, a ‘Renal Angina Index’ (RAI) scoring system has
recently been validated in children [8-10].
In this study, we aimed to determine the ability of
RAI, calculated on Day 0 of admission of children to an intensive care
unit, to predict the occurrence of severe AKI ( ³Stage
2 of Kidney Disease Improving Global Outcome (KDIGO) classification)
[11,12] on Day 3. We also compared the predictive ability of RAI with
other traditional markers of renal injury.
Methods
This was a prospective observational study, conducted
in the pediatric intensive care unit (PICU) of a tertiary care, public
hospital from January 2017 to October 2017. Ethical approval was
obtained from Institutional Ethics Committee, Maulana Azad Medical
College. All consecutive children, 1 month to 12 years of age, admitted
in PICU, with at least 8 hours of PICU stay and having documented
body-weight and intake-output records over this duration, were eligible
for the study. Known cases of chronic kidney disease, and children
already in stage 2 or 3 AKI or on dialysis were excluded.
Considering an incidence of severe AKI (stage 2 and
stage 3) in PICU as 10% per year [8], precision of 5%, and alpha error
of 0.05, a sample size of 144 was obtained. Expecting an attrition rate
of 10%, due to protocol deviation, our final sample size was 160.
After obtaining informed consent, subjects meeting
eligibility criteria were enrolled and relevant data including
anthropometry, demographic parameters, admission diagnosis,
co-morbidities, vital signs, and other clinical and laboratory
parameters were recorded. Those who had a PICU stay of less than 3 days
were excluded from the study.
Basic investigations like complete hemogram, urea,
creatinine, total protein, albumin, sodium, potassium were done on Day
0. Serum creatinine was estimated daily till Day 3, following which it
was done as per clinical requirement. The RAI was determined for all
enrolled subjects between 8 and 12 hours from the time of PICU admission
on Day 0. The Renal angina index was defined as the product of Risk
Group Score and Renal Injury Score [8]. Subjects having a RAI score
³8 on Day 0
were classified as RAI Positive.
Day 0 was defined as the first calendar day of PICU
admission, considered after a minimum of 8 hours from the time of PICU
admission. Day 3 was defined as the time period between 72 and 96 h
after PICU admission. Severe acute kidney injury was defined by the
KDIGO AKI classification stage ³2,
that is, serum creatinine of ³200%
above baseline/nadir value or £0.5
mL/kg/h of urine output for ³12
hours [11,12].
Fluid overload on Day 0 was determined by subtracting
urine output or any other major extra renal losses over 8-12 hours of
admission in PICU from the total fluid intake during this duration, and
was expressed as a percentage of bodyweight. The percentage fall in
estimated creatinine clearance (eCrCl) was calculated by comparing serum
creatinine at enrolment (after minimum 8-12 hours of PICU admission)
with the patient’s baseline serum creatinine (lowest serum creatinine
value documented in the 3 months prior to PICU admission), if available.
When baseline serum creatinine was not available, a reference eCrCl as
per age standards for GFR was used as a baseline GFR [13].
The primary outcome was the proportion of children in
level 3 PICU with Day 0 RAI score ³8
who develop severe AKI on Day 3 of admission. The secondary outcomes
were comparison of the predictive ability of Day 0 RAI with those of
serum creatinine and %fall in eCrCl on Day 0; the association of
different risk factors, with the development of severe AKI on Day 3.
Statistical analyses: Predictive ability of Day 0
RAI score was assessed by calculating sensitivity, specificity, positive
predictive value and negative predictive value. Receiver operating
characteristic (ROC) curves for Day 0 RAI values, Day 0 serum creatinine
and Day 0 percentage fall in eCrCl from baseline were constructed for
predicting severe AKI on Day 3. Other possible risk factors associated
with the occurrence of severe AKI on Day 3 were also assessed by
univariate and multivariate logistic regression. Risk factors associated
with mortality were also assessed by univariate and multivariate
analyses. The data were analyzed with SPSS version 23. All the results
were considered significant at P<0.05.
Results
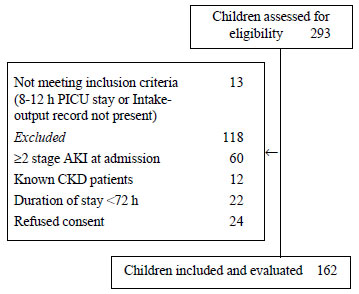
Out of a total of 293 children admitted in Level 3 of
PICU, during the study period, 162 children were enrolled (Fig.
1). Table I shows the baseline RAI scores; on Day 0,
86/162 (53%) children had a RAI ³8.
The lowest RAI of 1 was seen in 32 (19.8%) children, while 15 (9.3%) had
the highest RAI of 40.
 |
|
Fig. 1 Flow of participants in the
study.
|
TABLE I Classification of Children in PICU as Per Risk Group, Renal Injury and Renal Angina Index
|
Renal Angina Index parameter scores |
n (%) |
|
Risk Group Scores |
|
1 (PICU admission) |
92 (56.8) |
|
3 (Stem cell/solid organ transplantation) |
0 |
|
5 (Mechanical ventilation and use of inotropes) |
70 (43.2) |
|
Highest Renal Injury Scores
|
|
8
|
41 (25.3)
|
|
4
|
46 (28.4) |
|
2 |
33 (20.4)
|
|
1 |
42 (25.9) |
|
Renal Angina Index (RAI) |
|
Positive (>8) |
86 (53%) |
|
Negative (<8) |
76 (47%) |
|
PICU: Pediatric intensive care unit; Renal angina index:
Risk group score x Renal injury score.
|
The baseline characteristics of the enrolled children
and their outcomes have been compared between the groups of Positive and
Negative Day 0 RAI in Table II. Of the 86 children who
were RAI positive on Day 0, 62 (72.1%; 95% CI 62.6 %-81.4%) developed
severe AKI on Day 3 in contrast to 2/76 (2.6%) children who were RAI
negative (RR 95.5; 95% CI 21.7, 420.4; P <0.001). During their
entire PICU stay, a total of 66 out of 86 RAI positive children
developed severe AKI, the median (IQR) time for this development being 3
(2,4) days.
TABLE II Comparison of Parameters Between Children with Positive Day 0 RAI (RAI ≥8) and those with Negative RAI
|
Parameters |
RAI Positive
|
RAI Negative
|
|
(n=86) |
(n=76) |
|
*Age, mo (median, IQR) |
7 (3-24) |
24 (4-60) |
|
Gender (Male:Female) |
51:35 |
44:32 |
|
Major Diagnostic group, n (%) |
|
*Respiratory |
65 (76) |
41 (54) |
|
CNS |
20 (23) |
16 (21) |
|
Gastrointestinal |
14 (16) |
19 (25)
|
|
Sepsis |
19 (22) |
9 (12) |
|
PRISM 3 score, n(%)
|
|
<5 |
51
|
49 |
|
5-10 |
21 |
23 |
|
10-20 |
12 |
3 |
|
>20 |
2 |
1 |
|
At enrolment, n (%) |
|
*GCS
|
11.5 (2.5) |
12.3 (1.8) |
|
#Mechanical ventilation |
61 (71) |
17 (22) |
|
#Inotropes
|
67 (78) |
24 (32) |
|
Fluid overload (% body wt) |
1.3 (1.3) |
2.8 (1.6) |
|
$% Fall in eCrCl from baseline
|
39.8 (18.8) |
14.6 (18.3) |
|
Maximum AKI stage, n (%)
|
|
#No AKI |
1 (1.2) |
47 (61.8)
|
|
Stage 1 |
19 (22.1) |
26 (34.2) |
|
#Stage 2 |
51 (59.3) |
2 (26) |
|
#Stage 3 |
15 (17.4) |
1 (1.3) |
|
#Severe AKI (>Stage2) on Day 3 |
62 (72) |
2 (2.6) |
|
†Mortality, n (%) |
21 (24) |
6 (8) |
|
RAI: Renal angina index; CNS: Central nervous system; GCS:
Glasgow Coma Scale; eCrCl: estimated creatinine clearance; AKI:
acute kidney injury; PICU: Pediatric intensive care unit;
*P<0.005; #P<0.001; $P<0.01; †P=0.005. |
A positive Day 0 RAI was found to have a sensitivity
of 96.9%, a specificity of 75.5%, a positive predictive value of 72% and
a negative predictive value of 97.4%. A Receiver operating
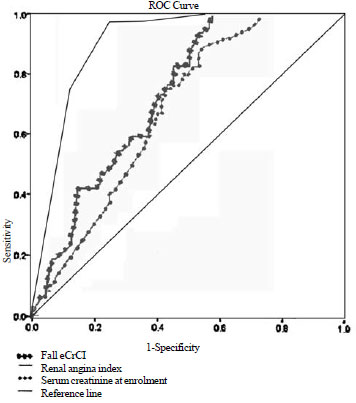
characteristic (ROC) curve was constructed for assessing individual
values of Day 0 RAI for predicting severe AKI on Day 3, with an AUC
(Area Under the Curve) of 0.90 (95% CI 0.85, 0.95). Serum creatinine at
enrolment and Percentage fall in eCrCl from baseline showed AUC (0.68
and 0.73, respectively) much inferior to that of RAI (Fig. 2).
 |
|
Fig. 2 Receiver Operating
Characteristic curves for (i) Day 0 Renal angina index, (ii) Day
0 serum creatinine, and (iii) percentage fall in eCrCl from
baseline on Day 0 for predicting severe AKI on Day 3.
|
Univariate regression analysis done to evaluate the
effect of individual parameters showed that use of mechanical
ventilation, inotropes, amikacin, hypotension, fall in eCrCl from
baseline, mean oxygen saturation during ICU stay and Day 0 positive RAI
score were significantly associated with occurrence of severe AKI on Day
3. Multivariate analysis showed that positive Day 0 RAI score was the
only parameter which had an independent association with the occurrence
of Severe AKI on Day 3 (Table III).
TABLE III Univariable and Multivariable Analysis Evaluating Association of Individual
Parameters with Occurrence of Severe Acute Kidney Injury on Day 3
|
Univariate analysis |
Multivariate analysis |
|
Parameters |
OR (95% CI); P value
|
OR (95% CI); P value |
|
Age (mo) |
0.9 (0.9, 1.0); 0.17 |
|
|
Gender |
1.2 (0.6, 2.2); 0.63 |
|
|
Sepsis |
1.7 (0.7, 3.8); 0.21 |
|
|
Mechanical ventilation
|
7.8 (3.8, 16.0); <0.001 |
2.2 (0.6, 8.0); 0.25 |
|
Use of amikacin |
1.9 (1.0, 3.8); 0.04 |
1.3 (0.5, 3.3); 0.58 |
|
Use of inotropes |
6.6 (3.1, 13.9); <0.001 |
1.1 (0.2, 5.3); 0.94 |
|
Hypotension |
3.9 (2.0, 7.7); <0.001 |
1.4 (0.4, 4.9); 0.62 |
|
Fall in estimated creatinine clearance from baseline
|
1.0 (1.0, 1.1); <0.001 |
1.0 (0.97, 1.04); 0.65 |
|
Urine output in 8 h prior to enrolment |
1.2 (0.9,1.4); 0.13 |
|
|
Fluid overload (% of body weight) |
1.1 (0.8, 1.3); 0.57 |
|
|
Episodes of significant hypoxia |
5.4 (1.7, 17.7); 0.005 |
|
|
Mean O2 saturation during ICU stay |
0.9 (0.8, 1.0); 0.024 |
0.9 (0.8, 1.0); 0.16 |
|
Post-operated case |
0.4 (0.1, 2.1);0.28 |
|
|
Positive Renal Angina Index score on day 0 |
95.6 (21.7, 420.5); <0.001 |
55.5 (8.9, 333.3); <0.001 |
|
PRISM III score ≥10 |
2.7 (0.99, 7.4); 0.53 |
|
Of the total of 69 children developing severe AKI
(³ Stage 2)
during ICU stay, 49 (71%) children had a complete recovery of renal
function and 4 had some improvement. On the other hand, 16 children had
persistent severe derangement of renal function and all of them died at
a median (IQR) time of 3.5 (2,5) days after admission.
The median (IQR) length of ICU stay for all enrolled
subjects was 6 (4,11) days, with no significant difference between those
who were RAI Positive or RAI negative [6 (4,13) vs 5 (4,9); P=0.54].
Discussion
This hospital-based study showed that a positive RAI
at admission was a strong predictor of AKI on day 3. RAI performed
better than baseline serum creatinine and percentage fall in eCr/Cl from
baseline in predicting AKI.
The study, comprising of children with a similar
spectrum of diseases, as observed in other studies in intensive care
[8,14-17], had a significantly higher proportion of younger children in
the RAI positive group, compared to the RAI negative group, in contrast
to the AWARE study [17]. However, other studies found neither
demographic parameters nor primary system of involvement to have any
significant bearing on renal angina positivity [18,19].
With a total of 91 (56%) children receiving one or
more inotropes, and 78 (48%) on mechanical ventilation, at enrolment,
our study had a relatively larger proportion (over 50%) of children in
PICU showing RAI positivity on Day 0, compared to other reports [8,19].
As reported by others, severity of sickness, reflected in higher PRISM
scores, was significantly more common in the RAI positive group [10,18].
However, unlike other studies, development of severe AKI was not
influenced by the severity of PRISM scores in our study. The
unreliability of the non-specific ‘severity of illness’ (SOI) scores
like PRISM or PIM (Pediatric Index of Mortality) to adequately predict
progression of individual organ failure is already a known fact in
medical literature [20,21].
In our study, more than 70% of RAI positive developed
severe AKI on Day 3; the proportion being higher than those reported by
others [18,19]. Basu, et al. [8], also concluded that a RAI under
8 had high NPV (92-99%) for Day-3 AKI. However, the predictive ability
of RAI in their study was only modest (AUC 0.78-0.81) [8], while another
study found an even poorer predictive accuracy of RAI (AUC 0.61-0.82)
[22].
Our results are in parallel with the results of the
AWARE study [17] and of Basu, et al. [8], wherein RAI
outperformed other markers of renal injury. Other authors have also
provided multivariate analysis showing RAI as an important marker of AKI
[22].
Our study has the limitation that while calculating
the percentage fall eCrCl on Day 0, we have used age based Western
reference standards for GFR as baseline GFR [13]. This may have
overestimated the RAI scores, but due to lack of standards available in
Indian children, this is the best approximation as of today.
This study shows that RAI, when
³8 on the first day
of hospitalization, reliably identifies those critically ill children
who are at higher risk for developing severe AKI on Day 3 of
hospitalization. The discriminative accuracy of RAI supersedes that of
individual traditional creatinine based renal injury parameters. The
independent effect of RAI for predicting severe AKI is maintained even
after adjustment for other risk factors. Intensive care practitioners
should consider using RAI for risk stratification and prognostication in
sick children.
Contributors: J: enrolled subjects, collected
data, was involved in analysis of data and creation of final draft. KM:
conceptualized the study, supervised and monitored the conduct of the
study, and was responsible for analysis of data and writing the final
manuscript; MK: provided critical inputs Meaning the study, supervised
the study and approved the manuscript writing; DS: was responsible for
regular guidance and supervision of data collection and approval of
final manuscript.
Funding: None; Competing interest: None
stated.
|
What is Already Known?
• Serum creatinine is
presently used as a marker of acute kidney injury.
What This Study Adds?
•
A positive Renal Angina Index (³8)
is a superior tool, compared to serum creatinine, to reliably
identify critically ill children at high risk for severe acute
kidney injury by day 3 of hospital admission.
|
References
1. Mammen C, Al Abbas A, Skippen P, Nadel H, Levine
D, Collet JP, et al. Long-term risk of CKD in children surviving
episodes of acute kidney injury in the intensive care unit: a
prospective cohort study. Am J Kidney Dis. 2012;59:523-30.
2. Askenazi DJ, Feig DI, Graham NM, Hui-Stickle
S, Goldstein SL, et al. 3-5 year longitudinal follow-up of
pediatric patients after acute renal failure. Kidney Int. 2006;69:184-9.
3. Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet
T, Gottesman R, et al. Acute kidney injury is an independent risk
factor for pediatric intensive care unit mortality, longer length of
stay and prolonged mechanical ventilation in critically ill children: A
two-center retrospective cohort study. Crit Care. 2011;15:R146
4. Waikar SS, Betensky RA, Bonventre JV. Creatinine
as the gold standard for kidney injury biomarker studies?Nephrol Dial
Transplant. 2009;24:3263-5.
5. Honore PM, Jacobs R, Joannes-Boyau O, Verfaillie
L, De Regt J, Van Gorp V, et al. Biomarkers for early diagnosis
of AKI in the ICU: Ready for prime time use at the bedside?. Ann
Intensive Care. 2012;2:24
6. Bagshaw SM, Zappitelli M, Chawla LS. Novel
biomarkers of AKI: The challenges of progress ‘Amid the noise and the
haste’. Nephrol Dial Transplant. 2013;28:235-8.
7. Hjortrup PB, Haase N, Wetterslev M, Perner A.
Clinical review: Predictive value of neutrophil gelatinase-associated
lipocalin for acute kidney injury in intensive care patients.Crit
Care. 2013;17:211
8. Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR,
Chawla LS, et al. Derivation and validation of the renal angina
index to improve the prediction of acute kidney injury in critically ill
children. Kidney Int. 2014;85:659-67
9. Goldstein SL, Chawla LS. Renal angina. Clin J Am
Soc Nephrol. 2010;5:943-9.
10. Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS,
Goldstein SL. Incorporation of biomarkers with the renal angina index
for prediction of severe AKI in critically ill children. Clin J Am Soc
Nephrol. 2014;9:654-62.
11. Kellum JA, Lameire N; KDIGO AKI Guideline Work
Group. Diagnosis, Evaluation, and Management of Acute Kidney Injury:
A KDIGO Summary (Part 1). Crit Care. 2013;17:204.
12. Khwaja A. KDIGO clinical practice guideline for
acute kidney injury. Nephron Clin Pract. 2012;120:c179-84.
13. Heilbron DC, Holliday MA, Al-Dahwi A, Kogan BA.
Expressing glomerular filtration rate in children. Pediatr
Nephrol.1991;5:5-11.
14. Naik S, Sharma J, Yengkom R, Kalrao V, Mulay A.
Acute kidney injury in critically ill children: Risk factors and
outcomes. Indian J Crit Care Med. 2014;18:129-33.
15. Mehta P, Sinha A, Sami A, Hari P, Kalaivani
M, Gulati A,et al. Incidence of acute kidney injury in
hospitalized children. Indian Pediatr. 2012;49:537-42.
16. Krishnamurthy S, Mondal N, Narayanan P, Biswal
N, Srinivasan S, Soundravally R, et al. Incidence and etiology of
acute kidney injury in southern India. Indian J Pediatr. 2013:183-9.
17. Basu, Rajit K, Kaddourah A, Goldstein SL, AWARE
group. Assessment of a renal angina index for prediction of
severe acute kidney injury in critically ill children: A multicentre,
multinational, prospective observational study. Lancet. 2017;2:112-20.
18. Menon S, Goldstein SL, Mottes T, Fei L, Kaddourah
A, Terrell T, et al. Urinary biomarker incorporation into the
renal angina index early in intensive care unit admission optimizes
acute kidney injury prediction in critically ill children: A prospective
cohort study. Nephrol Dial Transplant. 2016;3:586-94.
19. Kaur R, Dhooria GS, Pooni PA, Bhat D, Bhargava
S, Kakkar S, et al. Utilization of the renal angina index in PICU
of a developing country for prediction of subsequent severe acute kidney
injury. Pediatr Nephrol. 2018 Jul 9. [Epub ahead of print]
20. Basu RK, Chawla LS, Wheeler DS, Goldstein SL.
Renal angina: An emerging paradigm to identify children at risk for
acute kidney injury. Pediatr Nephrol. 2012;27:1067-78.
21. Fiaccadori E, Maggiore U, Lombardi M, Leonardi S,
Rotelli C, Borghetti A. Predicting patient outcome from acute renal
failure comparing three general severity of illness scoring systems.
Kidney Int. 2000;58:283-92.
22. Sethi SK, Raghunathan V, Shah S, Dhaliwal M, Jha
P, Kumar M, et al. Fluid overload and renal angina index at
admission are associated with worse outcomes in critically Ill children.
Front Pediatr. 2018;6:118.
|
|
|
 |
|

