|
|
|
Indian Pediatr 2019;56:643-646 |
 |
Levetiracetam versus
Phenobarbitone in Neonatal Seizures – A Randomized Controlled
Trial
|
|
Vykuntaraju K Gowda 1,
Ayesha Romana2,
Niranjan H Shivanna3, Naveen
Benakappa3 and Asha
Benakappa2
From Divisions of 1Pediatric Neurology and 3Neonatology,
2Department of Pediatrics, Indira Gandhi Institute of Child
Health, Bangalore, Karnataka, India.
Correspondence to: Dr Vykuntaraju K Gowda, Division of Pediatric
Neurology, Department of Pediatrics, Indira Gandhi Institute of Child
Health, Bangalore, Karnataka, India.
Email: [email protected]
Received: April 01, 2018;
Initial review: August 20, 2018;
Accepted: June 20, 2019.
Trial Registration: Clinical Trial Registry of India
(CTRI/2018/04/013161).
|
Objective: To compare the efficacy and safety of
intravenous Levetiracetam and Phenobarbitone in the treatment of
neonatal seizures. Design: Open labelled, Randomized controlled
trial. Setting: Level III Neonatal Intensive Care Unit (NICU).
Participants: 100 neonates (0-28 days) with clinical
seizures. Intervention: If seizures persisted even after
correction of hypoglycemia and hypocalcemia, participants were
randomized to receive either Levetiracetam (20 mg/kg) or Phenobarbitone
(20 mg/kg) intravenously. The dose of same drug was repeated if seizures
persisted (20 mg/kg of Levetiracetam or 10 mg/kg of Phenobarbitone) and
changeover to other drug occurred if the seizures persisted even after
second dose of same drug. Main outcome measures: Cessation of
seizures with one or two doses of the first drug, and remaining
seizure-free for the next 24 hours. Results: Seizures stoped in
43 (86%) and 31 (62%) neonates in Levetiracetam and Phenobarbitone
group, respectively (RR 0.37; 95%CI 0.17, 0.80, P<0.01). 10
neonates had adverse reactions in the phenobarbitone group (hypotension
in 5, bradycardia in 3 and requirement of mechanical ventilation in 2
neonates) while none had any adverse reaction in Levetiracatam group.
Conclusion: Levetiracetam achieves better control than
Phenobarbitone for neonatal seizures when used as first-line
antiepileptic drug, and is not associated with adverse drug reactions.
Keywords: Antiepileptic drugs, Convulsions, Management,
Neonate, Outcome.
|
|
S
eizures are the most common manifestation of
neurological insult during the neonatal period [1]. Etiology and
presentation of neonatal seizures are different from the children and
adults. The most common cause of symptomatic neonatal seizures is
hypoxic/ischemic encephalopathy (HIE) which affects approximately
1-2/100 live births [2,3]. There are no evidence-based guidelines for
the pharmacologic treatment of neonatal seizures and management is
highly variable. Phenobarbitone (PB) is the mainstay for neonatal
seizures treatment. The efficacy of PB in the complete resolution of
seizures varies between 33 and 77% [4]. Phenobarbitone can cause
neuronal apoptosis in vitro and have highly variable pharmacokinetics in
neonates [5-7].
Levetiracetam (LEV) may have a better safety profile
since it does not cause neuronal apoptosis in infant rodents [8]. A
recent review on the use of LEV in neonatal seizures revealed that
complete or near complete seizure cessation was achieved in 77% of LEV,
compared to 46% in PB group [9,10]. Literature pertaining to use of
levetiracetam in neonatal seizures is limited, and there is a lack of
randomized controlled trials. Hence we conducted this study with the
objective to compare the efficacy and adverse effects of LEV and PB in
the treatment of neonatal seizures.
Methods
This randomized controlled trial was conducted in the
level III NICU of a tertiary-care center over a period of 18 months
(November 2014 to April 2016). Outborn neonates (age 0-28 d) with
clinical seizures were enrolled in the study. Neonatal seizures were
clinically defined as abnormal, stereotyped and paroxysmal dysfunction
in the central nervous system (CNS), occurring within the first 28 days
after birth in full-term infants or before 48 weeks of gestational age
in preterm infants [11]. Neonates with hypoglycemia, hypocalcemia,
hypomagnesemia, those who received anticonvulsants prior to enrolment,
and those with major congenital malformations e.g., congenital
heart defects, neural tube malformations, diaphragmatic hernia,
choanal atresia, esophageal atresia, tracheoesophageal fistula,
omphalocele, gastroschisis, intestinal obstruction and imperforate anus)
were excluded.
Clinical details, seizure types and antiepileptic
administration, including the sequence of drugs, dosage, timing and
duration of therapy were recorded. Investigations included blood
glucose, serum calcium, magnesium, electrolytes, complete blood counts,
C- reactive protein, liver function tests, renal function tests,
arterial blood gas, lactate, ammonia, cranial ultrasono-graphy, Imaging
of brain, Electroencephalo-graphy (EEG), and metabolic and genetic
testing, whenever required to find out the cause for seizures.
Neonates with clinical seizures were randomly
assigned to receive either PB or LEV with a 1:1 allocation as per a
computer-generated randomization schedule and using sequentially
numbered, opaque and sealed envelopes. When an eligible neonate was
eligible to be enrolled, the envelope was opened by a clinician who was
not part of the study.
After ensuring patency of the airway, breathing and
circulation, blood sugar and ionic calcium level were performed. If
seizures persisted even after correction of hypoglycemia and
hypocalcemia, neonates were randomized for intervention to receive
either LEV (20 mg/kg) or PB (20 mg/kg) intravenously. Levetiracetam was
diluted in normal saline to achieve a concentration of 20 mg/mL and
administered intravenously at a rate of 1 mg/kg/min under
cardiorespiratory monitoring. If seizures terminated, LEV was continued
as maintenance at 20 mg/kg/day in two divided doses. If seizures
continued, another loading dose of LEV (20 mg/kg) was injected, and if
seizures still persisted, patient was switched over to PB. PB was
administered in the dose of 20 mg/kg diluted in 1: 10 normal saline
given intravenously slowly at the rate of 1 mg/kg/min under
cardiorespiratory monitoring; if seizures were terminated, it was
continued at 5 mg/kg/day in two divided doses as maintenance. Another
loading dose of 10 mg/kg of PB was administered in neonates who failed
to respond, and if seizures still persisted after two loading doses,
patient was switched over to LEV.
The proportion of patients achieving cessation of
seizures following the first or second dose of the drug (PB or LEV), and
those remaining seizure-free for next 24 hours was considered as the
primary outcome. Secondary outcome measure was the proportion of
patients experiencing adverse events. Termination of seizure was defined
clinically if there were no abnormal movement/eyeball deviation/nystagmus,
no change in heart rate, no change in respiration/saturation and
autonomic dysfunction. Adverse effects occurring within two hours of
drug administration, including desaturation, reduced respiratory rate,
increased ventilator support require-ment, arrhythmias, blood pressure,
or heart rate fluctuations by more than 10% compared to the previous 2
hours, or if vasopressors were initiated or increased, were recorded.
Increased ventilator requirement was considered, if requirement of tidal
volume more than 6 mL/kg on volume controlled-ventilator, peak
inspiratory pressure (PIP) more than 22 cm H 2O
in preterm and 23 cm H2O in
term, and mean airway pressure (MAP) of more than 12 cmH2O
on a pressure-controlled ventilator.
Informed consent was obtained from the parents on
pre-structured proforma as soon as possible after assessing for
eligibility. The study was approved by the institutional ethics
committee of Indira Gandhi Institute of Child Health, Bangalore. The
sample size required for this study was calculated as 100 (50 in each
group) with 95% two-sided significance ( a),
80% power, 1:1 randomization and a drop out of 15% assuming a difference
in proportion of outcomes between the groups as 31% (LEV 77% and PB 46%)
[12,13].
Statistical analyses: Continous variables were
compared between the two groups using independent samples t-test.
Termination of seizures at 24 hours and occurrence of adverse events
were compared by Chi-square test. Effect size and its 95% CI were
computed for the primary and secondary outcomes. P value of less
than 0.05 was considered as significant. The analyses were carried out
using the Statistical Package for Social Sciences (SPSS) 20.0 software.
Results
A total of 122 babies with clinical seizures were
assessed for eligibility during the study period; 22 were excluded and
50 neonates were randomized to each group (Fig. 1).
Baseline characteristics were comparable in the two groups (Table
I). The commonest etiology for seizures was hypoxic-ischemic
encephalopathy (HIE). Focal clonic seizures constituted the most common
type of seizure in the study population.
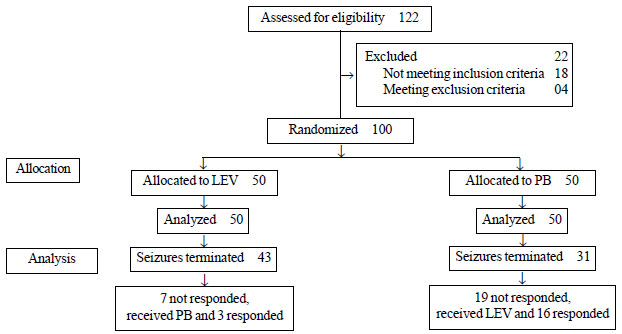
PB: Phenobarbitone; LEV: Levetiracetam
|
|
Fig. 1 Flow of participants in the study.
|
TABLE I Comparison of Baseline Characteristics of the Study Groups
|
Characteristics |
Levetiracetam |
Phenobarbitone |
|
(n=50) |
(n=50) |
|
Age (d), mean (SD) |
9.8 (8.50) |
8 (8.33) |
|
Male, n (%) |
28 (56) |
28 (56) |
|
Mode of delivery, n (%) |
|
Vaginal |
35 (70) |
36 (72) |
|
Caesarian |
15 (30) |
14 (28) |
|
Gestation, n (%) |
|
Term |
40 (80) |
42 (84) |
|
Preterm |
10 (20) |
08 (16) |
|
Birth weight (kg) , mean (SD) |
2.56 (0.64) |
2.73 (0.64) |
|
Etiology of seizures, n (%) |
|
HIE |
20 (40) |
24 (48) |
|
Neonatal sepsis/Meningitis |
18 (36) |
15 (30) |
|
Intracranial hemorrhage |
3 (6) |
2 (4) |
|
Benign neonatal epilepsy syndrome |
2 (4) |
1 (2) |
|
Malignant neonatal epilepsy syndrome |
1 (2) |
1 (2) |
|
Cortical malformation |
1 (2) |
1 (2) |
|
IEM |
1 (2) |
2 (4) |
|
Unknown |
4 (8) |
4 (8) |
|
HIE: Hypoxic ischemic encephalopathy, IEM: Inborn
errors of metabolism. |
Following first dose of drug, seizures stopped in 30
(60%) neonates in LEV group, and 25 (50%) neonates in PB group. In the
LEV group, there was a cessation of clinical seizures (and remaining
seizure free at 24 h) in 43 (86%), and in the PB group, it was 31 (62%)
after one or two doses (P<0.001). Seizure control was better (RR
0.37; 95% CI (0.17, 0.80) in the LEV group.
A total of 10 adverse events were observed in the PB
group and none in LEV group. Various adverse events noted in the PB
group were; hypotension in 5 neonates, bradycardia in 3 neonates and
requirement of mechanical ventilation in 2 neonates.
Three out of the seven neonates who did not respond
to LEV, responded to PB. Among the 19 neonates who did not respond to
PB, 16 showed seizure cessation with LEV. In the LEV group, 47 were
discharged, two left against medical advice, and one died. In the PB
group, 46 neonates were discharged and four left against medical advice.
Discussion
In the present study, we documented better
anticonvulsant efficacy and safety of LEV in comparison to PB as a
first-line antiepileptic drug in neonatal seizures. A higher proportion
of neonates had a cessation of seizures in LEV group as compared to PB
group. There were no adverse drug reactions noted in the neonates who
received LEV in the present study whereas, 10 of the neonates in the PB
group developed adverse drug reactions. The efficacy of LEV has been
earlier demonstrated in a study by Ramantani, et al. [13]
, in which 30 (78%) out of 38 infants were
seizure-free after receiving LEV. In study by Khan, et al. [14],
19 (86%) of the 22 neonates
demonstrated seizure cessation within 1 hour of administration. In a
systematic review of the efficacy of LEV in neonatal seizures, complete
or near-complete seizure cessation was achieved in 37/48 (77%) who
received LEV as first-line drug, and 24/52 (46%) of the ones with PB as
first-line AED [10]. These results show that LEV is at least as
effective as PB in the control of neonatal seizures as a first-line
agent. However, in a study by Abend, et al. [15], LEV was
associated with seizure improvement within 24 hours in only 8 (35%) of
23 neonates. The low response to LEV in this study could be due to the
usage of LEV as first-line anti-epileptic drug in only one neonate in
the study. Few other studies [10,16] have documented good seizure
control with LEV when it was used as a second- or third-line agent in
control of neonatal seizures. The safety of LEV in neonates has also
been documented in previous studies [13,15-17].
The limitation of our study was that we did not
perform electroencephalographic monitoring to document cessation of
seizure activity. However, in most of the neonatal units, especially
with limited resources, clinical control of seizures is usually the only
guide to treatment. Thus, despite this limitation, the generalizability
of our study for such settings is reasonable. Lack of long-term
follow-up and inability to perform therapeutic drug levels of PB and LEV
were the other limitations of the present study. The sample size of our
study was also inadequate for the outcomes related to various adverse
effects.
We conclude that levetiracetam is an effective and
safer alternative to phenobarbitone in the management of neonatal
seizures, as a first-line AED.
Contributors: VR: conceptualization of study, and
critical inputs to manuscript for important intellectual content; AR:
data collection and writing the manuscript; NS and NB: data collection
and analysis, and critical inputs to manuscript writing; AB: supervision
of the work and revision of manuscript.
Funding: None; Competing interests:
None stated.
|
What is Already Known?
•
Phenobarbitone currently
represents the antiepileptic drug of choice in the management of
neonatal seizures.
What this Study Adds?
•
Levetiracetam is an effective and safer alternative to
phenobarbitone as a first line drug in the management of
neonatal seizures.
|
References
1. Thibeault-Eybalin MP, Lortie A, Carmant L.
Neonatal seizures: Do they damage the brain? Pediatr Neurol.
2009;40:175-80.
2. Ronen GM, Penney S, Andrews W. The epidemiology of
clinical neonatal seizures in Newfoundland: a population-based study. J
Pediatr. 1999;134:71.
3. Saliba RM, Annegers JF, Walker DK, Tyson JE,
Mizrahi EM. Incidence of neonatal seizures in Harris County, Texas,
1992-1994. Am J Epidemiol. 1999; 150:763.
4. Van Rooij LG, Hellström-Westas L, deVries LS.
Treatment of neonatal seizures. Semin Fetal Neonatal Med.
2013;18:209-15.
5. Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR,
Ivacko JA, et al. Neonatal seizures: multicenter variability in
current treatment practices. Pediatr Neurol. 2007;37:85-90.
6. Wheless JW, Clarke DF, Arzimanoglou A, Carpenter
D. Treatment of pediatric epilepsy: European expert opinion, 2007.
Epileptic Disord. 2007;9:353-412.
7. Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z,
Gardiner JC, et al. Phenobarbital compared with phenytoin for the
treatment of neonatal seizures. N Engl J Med. 1999;341:485-9.
8. Manthey D, Asimiadou S, Stefovska V, Kaindl AM,
Fassbender J, Ikonomidou C, et al. Sulthiame but not
levetiracetam exerts neurotoxic effect in the developing rat brain. Exp
Neurol. 2005;193:497e503.
9. Mruk AL, Garlitz KL, Leung NR. Levetiracetam in
neonatal seizures: A review. J Pediatr Pharmacol Ther. 2015;20:76-89.
10. McHugh DC, Lancaster S, Manganas LN. A systematic
review of the efficacy of levetiracetam in neonatal seizures.
Neuropediatrics. 2018;49:12-17.
11. Han JY, Moon CJ, Youn YA, Sung IK, Lee IG.
Efficacy of Levetiracetam for neonatal seizures in preterm infants. BMC
Pediatr. 2018;18:131.
12. Boylan GB, Rennie JM, Pressler RM, Wilson G,
Morton M, Binnie CD. Phenobarbitone, neonatal seizures, and video EEG.
Arch Dis Child Fetal Neonatal Ed. 2002;86:165-70.
13. Ramantani G, Ikonomidou C, Walter B, Rating D,
Dinger J. Levetiracetam: Safety and efficacy in neonatal seizures. Eur J
Paediatr Neurol. 2011;15:1-7.
14. Khan O, Chang E, Cipriani C, Wright C, Crisp E,
Kirmani B. Use of intravenous levetiracetam for management of acute
seizures in neonates. Pediatr Neurol. 2011;44:265-9.
15. Abend NS, Gutierrez-Colina AM, Monk HM, Dlugos
DJ, Clancy RR. Levetiracetam for treatment of neonatal seizures. J Child
Neurol. 2011;26:465-70.
16. Rakshasbhuvankar A, Rao S, Kohan R, Simmer K,
Nagarajan L, J Clin Neurosci. 2013;20:1165-7.
17. Koppelstäetter A, Buhrer C, Kaindl AM. Treating
neonates with levetiracetam: A survey among German university hospitals.
Klin Pediatr. 2011;223:450-2.
|
|
|
 |
|

