|
Clinicopathological conferences |
|
|
Indian Pediatr 2018;55:693-697 |
 |
An Infant with
Respiratory Distress and Loose Stools
|
|
Poojitha Reddy 1,
Devika Laishram2,
Ankur Kumar Jindal2,
Kirti Gupta3 and
Amit Rawat2
From 1Department of Pathology, 2Allergy
Immunology Unit, Department of Pediatrics, and 3Department of
Histopathology, Post Graduate Institute of Medical Education and
Research, Chandigarh, India.
Correspondence to: Dr Kirti Gupta, Professor, Department of
Histopathology, Post Graduate Institute of Medical Education and
Research (PGIMER), Chandigarh 160 012, India.
Email:
[email protected]
|
We present the case of a 3-month-old girl who was
admitted with complaints of loose stools and respiratory distress. She
also had a history of rash and alopecia. Laboratory investigations
revealed lymphopenia with reduced immunoglobulin G and immunoglobulin A.
Lymphocyte subset analysis by flow cytometry revealed T-B+NK+ severe
combined immunodeficiency (SCID). She died due to severe pneumonia,
shock and pulmonary hemorrhage. Autopsy findings revealed disseminated
cytomegalovirus infection in the lung, liver, adrenals and heart. Thymus
was found to be dysplastic and showed characteristic histopathologic
features of SCID.
Keywords: Alopecia, Cytomegalovirus, Diarrhea, Lymphopenia,
Pneumonia, Severe combined immunodeficiency.
|
|
Clinical Protocol
History: A 3-month-old girl, product of 3rd
degree consanguineous marriage presented with history of generalized,
erythematous, maculopapular rash all over body that developed at one
month of age. Rash was associated with skin peeling and it resolved
spontaneously in one month. She subsequently had progressive loss of
scalp hair and eyebrows. She also had bilateral purulent ear discharge
since the age of 2 months associated with cough, respiratory distress
and loose stools. Child was admitted twice for these complaints in a
nearby hospital and was managed with IV antimicrobials. She had received
BCG vaccination at 1 month of age, however, she developed no reaction at
the BCG site.
Clinical examination: She had tachycardia (heart
rate 150/minute), tachypnea (respiratory rate-62/minute), pallor,
alopecia with loss of eyebrows (Fig. 1), skin peeling in
the left axilla, no BCG scar, coarse crepitation in bilateral chest and
hepato-splenomegaly. Initial clinical possibilities were Human
Immunodeficiency Virus (HIV) infection, Severe Combined Immunodeficiency
(SCID) with maternal engraftment, congenital Cytomegalovirus (CMV)
infection and Langerhans cell histiocytosis.
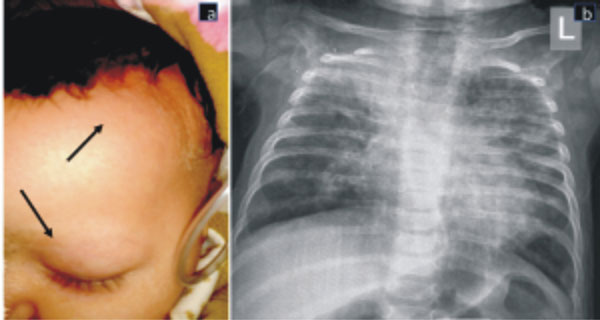 |
| (a) |
(b) |
|
Fig. 1 Alopecia over left fronto-parietal
region and loss of eyebrows (arrows) (a); chest X-ray (anteroposterior
view) showing interstitial pattern of infiltrates in both lungs,
predominantly perihilar. Homogenous opacity in right upper lung
fields and absent thymic shadow (b).
|
Laboratory investigations: She had anemia
(hemoglobin- 83 gm/L), lymphopenia (white cell count 16.3×10 9/L,
differential counts P79L16M5,
absolute lymphocyte counts 2.6×109/L),
transaminitis (alanine aminotransferase 94 IU/L and aspartate
aminotransferase 303 IU/L), and a high C-reactive protein (39.6 mg/L).
Serial arterial blood gas analysis revealed hypoxemia and hypercarbia.
Chest X-ray revealed bilateral non-homogeneous opacities
predominantly distributed in the peri-hilar region. (Fig. 1b).
Ultrasound abdomen showed ileo-ileal intussusception. Contrast enhanced
Computed Tomography (CECT) chest revealed multiple bilateral pulmonary
nodules with consolidations. Human immunodeficiency virus (HIV) serology
was non-reactive, Fine needle aspiration cytology from axillary lymph
node showed reactive lymphoid hyperplasia, gastric lavage for acid fast
bacilli and pneumocystis jiroveci stain was negative, serum
galactomannan index was elevated (5.8, normal <0.5), blood culture was
sterile, IgM Cytomegalovirus (CMV) serology and CMV DNA PCR was
positive.
Immunological investigations (Table I):
She had undetectable IgG and IgA and normal IgM; nitroblue tetrazolium
(NBT) dye reduction test and dihydrorhodamine 123 (DHR) assay were
normal; lymphocytes subsets showed reduced CD3+ T cells (32% of all
lymphocytes), normal CD19+ B cells (29% of all lymphocytes) and normal
CD56+ NK cells (39% of all lymphocytes); CD 127 (IL-7Rá) expression was
reduced (Web Fig.1a and 1b); CD45RA
expression on CD3+T cells and CD8+T cell was reduced suggestive of low
naďve T cells; CD45RO expression on CD3+T cells and CD8+T cells was
elevated suggestive of increased memory T cells (likely to be of
maternal origin).
TABLE I Laboratory Investigations of Index Case
|
Investigation |
Results |
Normal reference
|
|
|
range |
|
IgG (mg/dL) |
<201 |
240-880 |
|
IgA (mg/dL) |
<17 |
10-50 |
|
IgM (mg/dL) |
90 |
20-100 |
|
CD3+ T cells |
32.68% |
51-77.6% |
|
CD19+ B cells |
29.79% |
11-41% |
|
CD16/56+ NK cells |
33.41% |
3-14% |
|
CD45RA expression on CD3+T cells
|
2.23% |
67.83% |
|
CD45RO expression on CD3+T cells |
94.7% |
31.18% |
|
CD45RA expression on CD8+T cells |
5.18% |
74.54% |
|
CD45RO expression on CD8+T cells |
93.6% |
22.35% |
|
CD-127 (IL-7Ra) expression on CD3+ |
16.5% |
53.95%
|
|
T lymphocytes |
|
(normal control) |
Course and management: Child was administered
intravenous ceftriaxone and cloxacillin, however, antimicrobials were
upgraded to vancomycin, imipenem, cotrimoxazole, amphotericin B and
anti-tubercular therapy (ATT). ATT was initiated empirically as the
child had received BCG vaccination at birth and pneumonia could be a
manifestation of disseminated BCG disease [1]. Oxygen therapy was
administered through nasal prong oxygen initially but on 6 th
day of hospital stay, child was intubated and kept on manual
intermittent positive pressure respiration. She was also given 5 grams
of intravenous immunoglobulin (IVIG); fluid bolus and inotropes
(dopamine and dobutamine) for shock and irradiated packed cell
transfusion. She also developed pneumothorax requiring intercostal
drainage tube and massive pulmonary hemorrhage leading to cardiac arrest
and death.
Unit’s final diagnosis: Severe combined
immunodeficiency (T-B+NK+) with IL-7R alpha deficiency with maternal
engraftment, severe pneumonia, sepsis, septic shock and pulmonary
hemorrhage.
Discussion
Clinical discussant: We have a 3-month-old girl
with history of consanguinity presenting with rash, alopecia, skin
peeling, otitis media, pneumonia, loose stools and intussusception. She
had anemia, lymphopenia, transaminitis, elevated galactomannan index,
positive CMV DNA PCR, multiple pulmonary nodules on CT chest, low
immunoglobulins, T-B+NK+ phenotype on lymphocyte subsets, low naďve T
cells and reduced IL-7R a
expression on T cells.
Case analysis can be discussed with respect to the
underlying disease; the etiology for pneumonia; the cause for
Intussusception; and the terminal events.
The clinical presentation is suggestive of an
underlying immune defect. After ruling out HIV infection, the most
likely underlying diagnosis is SCID, as she was born to a
consanguineously married couple and presented within the first 2 months
of age with severe pneumonia, otitis media, alopecia (likely due to
maternal engraftment), and absence of BCG scar. Laboratory
investigations revealed lymphopenia, hypogamma-globulinemia, low CD3+ T
cells, naďve T cells and absence of thymic shadow on chest X-ray.
She fulfills the European society of immunodeficiency (ESID) registry
working definition for clinical diagnosis of SCID.
SCID has four phenotypes based on the presence or
absence of B and NK cells (T-B-NK+; T-B-NK-; T-B+NK-; T-B+NK+). Our
child had T-B+NK+ phenotype and she also had reduced expression of IL-7R a.
She had features of maternal engraftment (i.e.
presence of maternal T lymphocytes in the circulation leading to
manifestations that are like graft versus host disease). Most common
manifestation of maternal engraftment is skin involvement with eczema,
erythema, alopecia and skin peeling; followed by liver involvement (with
mild to moderate elevation of liver enzymes) and gastrointestinal tract
involvement (diarrhea). Index child has history of generalized erythema
and peeling of skin and alopecia. She also had elevated liver enzymes.
The etiology for pneumonia is likely to be
polymicrobial. Invasive fungal infection can be considered in view of
positive serum galactomannan index, CMV can be considered in view of
positive CMV DNA PCR in the blood and suggestive radiological findings.
Pneumocystis jiroveci pneumonia can also be considered in view of
radiological findings. As she received BCG vaccination, so a possibility
of disseminated BCG infection with pneumonia can also be considered.
She also had intussusception as evident on ultrasound
of the abdomen. The intussusception in this age group would most likely
be related to infections which could be a viral or bacterial infection.
In the index child, Mycobacterium bovis and CMV can be
considered. BCG infection leading to recurrent intussusception has
previously been reported in SCID [2].
The cascade of terminal event can be summarized as
SCID leading to severe pneumonia and polymicrobial sepsis that has led
to multiorgan dysfunction and pulmonary hemorrhage finally causing shock
and death.
Therefore, the final diagnosis is severe combined
immunodeficiency (T-B+NK+ phenotype with IL-7R a
deficiency) with pneumonia (CMV, Aspergillus, Pneumocystis jiroveci),
pulmonary hemorrhage and intussusception (Mycobacterium bovis or
CMV)
Senior resident of the treating unit: The
diagnosis of SCID is not in doubt here and other differentials diagnosis
that were considered can easily be excluded based on the immunological
investigations. I expect severe depletion of T cells in the thymus;
changes of GVHD in skin and gut and CMV or Mycobacterium bovis in
lungs and gastrointestinal tract.
Physician 1: Was fundus checked in the index case
to look for evidence of CMV retinitis?
Senior resident of the treating unit: The fundus
evaluation was done and there was no evidence of CMV retinitis.
Pediatrician 1: Mycobacterium bovis
infection secondary to BCG vaccination often produces skin lesions and
fine needle aspiration from these skin lesions can yield acid fast
bacilli (AFB). However, there were no skin lesion in the index child.
Gastric lavage for AFB was negative. However, it is not always necessary
to find AFB on gastric lavage. Index child had alopecia and loss of
eyebrows that was suggestive of maternal engraftment or Omenn syndrome.
Pediatrician 2: The syndrome of SCID should be
suspected if the child presents within the first few months of life with
rash and polymicrobial sepsis which is not responding to antimicrobials.
If hemogram shows lymphopenia as in the index child, SCID should be
considered. The phenotype of SCID can be evaluated with the help of flow
cytometry. Index child had an unusual phenotype i.e. T-B+NK+.
Pathology Protocol
A complete autopsy was performed on this 3-month-old
girl. The peritoneal cavity revealed ascites with 270 mL of
straw-colored fluid. The other serous cavities were within normal
limits.
Thymus weighed 2 g (normal weight for this age is 3
g) and revealed maintained lobular architecture on gross examination.
However, on microscopy cortico-medullary distinction was lost and there
was absence of Hassall’s corpuscle. These features are consistent with
thymic dysplasia. CD3 immunostain revealed reduction in T-lymphocytes,
however, the thymocytes were preserved as highlighted by pan-cytokeratin
(Web Fig. 2 a-d).
All sampled lymph nodes showed depletion of lymphoid
population. T cell zones i.e. paracortical and interfollicular regions
showed reduction in T lymphocytes as highlighted by CD3. CD20 performed
revealed normal population of B lymphocytes (Web Fig. 2e-g).
Peyer’s patches were hypertrophic with relative preservation of B cell
zone (Web Fig. 2h). However, there was marked
reduction of T cell zone. Spleen weighed 20 g (normal for this age is 25
g). On histology, there was mild congestion of the red pulp with
preservation of B cell zone and relative reduction of periarteriolar T
cell zone.
Both the lungs were heavy (weight 250 g) with dull
pleural surface. Many subpleural nodules were identified. The cut
surface showed diffuse areas of hemorrhagic consolidation in all the
lobes, bilaterally. Tracheo-bronchial tree was unremarkable.
Microscopically, there was interstitial widening with mild
lymphomononuclear infiltrate along with many large cells protruding into
the alveolar lumen. Many cytomegalovirus inclusions (CMV) were noted
within alveolar pneumocytes and endothelial cells. These cells
demonstrated nucleomegaly along with presence of basophilic to
amphophilic inclusions in the nucleus and a perinuclear halo (Web
Fig. 2i). Immunostaining for CMV revealed the burden of
infection. Similar inclusions were also noted in the bronchial
epithelial cells and endothelial cells of blood vessels. Adjacent areas
of lung parenchyma showed diffuse alveolar hemorrhages and focal hyaline
membrane formation. No granulomas were identified and Ziehl-Neelsen
stain for acid fast bacilli (AFB) was negative. PAS stain performed did
not reveal any fungal hyphae and gram’s stain performed did not reveal
any micro-organisms.
Adrenals examined showed foci of necrosis with the
presence of CMV inclusions within the necrotic foci. Heart (weight 30 g)
was unremarkable grossly. However, few endothelial cells with the
interstitial vessels in the myocardium showed presence of CMV inclusion
which was confirmed on immunohistochemistry. Liver (weight 450 g) was
unremarkable on gross examination. Cut surface was pale yellow to greasy
in appearance. On microscopy, diffuse macrovesicular steatosis
predominantly involving the zone 1 and zone 2 areas with relative
preservation of zone 3 hepatocytes was noted. Few scattered CMV
inclusions were also noted in the Kupffer cells of the sinusoids which
was confirmed on immunohistochemistry. Rest of the organs including
brain, kidneys, bone marrow, stomach, esophagus, skin, thyroid, skeletal
muscle were unremarkable grossly and microscopically.
Final autopsy diagnosis:
• Thymic dysplasia
• Disseminated CMV infection – Lung
(predominant), Adrenal, Liver, Heart
• Macrovesicular steatosis
• Intussusception of small intestine
Senior resident of the treating unit: There is
perfect correlation between flow cytometry reports and pathology
findings in lymph nodes. B cells were present in the flow cytometry
report and were also found in the lymph nodes. In many types of SCID,
the B cells are absent in blood and there we see depletion of B cell
zone in the lymph nodes. Was any organism isolated at the site of
intussusception?
Pathologist 1: No organism was seen at the site
of intussusception. The intussusception was likely due to hyperplasia of
Peyer’s patches.
Physician 1: Was flow cytometry analysis done for
the parent’s sample and were parents counselled about future
pregnancies.
Senior resident of the treating unit: Flow
cytometry analysis of parent’s sample is not usually done in cases of
SCID. Molecular analysis will be done for both child and parents.
Parents have been advised to avoid further pregnancy till the genetic
confirmation, following which a prenatal diagnosis will be offered.
Pediatrician 3: This is an autosomal recessive
SCID and parents are likely to be the carriers. So, flow cytometry is
not useful in diagnosing carrier status which can only be diagnosed with
molecular analysis.
Pathologist 2: In the year 1980s, these types of
cases did not fit into any clinical diagnosis, these infants will have
infections like CMV, PCP with hypogammaglobulinemia and lymphopenia.
However, the level of investigations was not as extensive as they are
now. One thing that was pursued extensively at that time was the
possibility of AIDS. Extensive investigations were done on thymus and
lymphoid system. Serial sections of thymus were done which demonstrated
isolated Hassall’s corpuscles and normal blood vessels. In thymic
dysplasia thymus are abnormally small with small blood vessels, lobules
are shrunken and no lymphoid tissue in cortex and medulla which are
classical of SCID.
Pathologist 1: Lymphoid cells in the thymus were
due to maternal engraftment and it is difficult to differentiate
lymphoid cells from maternal origin or from the child. CMV infection is
most likely acquired as the major bulk of CMV disease was in lung and
not in liver.
Discussion
SCID is the most severe form of combined
immunodeficiency with an estimated prevalence of 1 in 58,000 live births
[3]. It is characterized by a block in T lymphocyte differentiation and
variable depletion of B and NK cells depending on the subtype of SCID
[4,5]. There are four major subtypes of SCID based on presence or
absence of B or NK cells. The index patient had T-B+NK+ SCID. This can
be caused by mutation in IL-7R a
or CD45 gene. Index patient had reduced expression of IL-7Ra.
SCID due to mutation in IL-7Ra
gene accounts for approximately 10% of all cases [6].
Children with SCID often present with recurrent
infections with an onset before the age of 6 months. Oral candidiasis,
persistent diarrhea, pneumonia and failure to thrive are the most common
presenting manifestations. There are no major differences between
various subtypes of SCIDs in their initial presentation; however, ADA
deficiency SCID tends to have severe lymphopenia (<0.5×10 9/L)
and hence very early and severe presentation. Common opportunistic
organisms seen in these patients are Pneumocystis jiroveci,
Aspergillus sp. and Cytomegalovirus. Majority of children die within
the first 2 years of life if not treated adequately. Respiratory tract
infections are common in patients with SCID and CMV pneumonia may be the
first presenting manifestation [7]. A persistent CMV respiratory
infection may be clinically or radiologically indistinguishable from
other respiratory viral infections. Index patient had interstitial
infiltrates on chest radiograph and positive CMV DNA PCR in blood and
autopsy revealed CMV infection in lung as well as in liver, heart and
adrenal glands.
In almost all subtypes of SCID, thymus is usually
atrophic [8] and poorly visualized on a chest radiograph [9] as was seen
in the index patient also. The histopathology of thymus in SCID
demonstrates depletion of lymphocytes and lack of differentiation of
thymic epithelium. Poor differentiation of thymic epithelium is
responsible for the absence of Hassall’s corpuscles and is on one of the
most important histopathological feature of SCID [10]. Thymic epithelium
has very important role in the normal differentiation of T-cell
population and due to thymic dysplasia in SCID, T-cell differentiation
is impaired [11].
Intussusception can have variable etiology (i.e.
viral, bacterial or protozoal infections and benign or malignant
tumors). As compared to adults in whom a definite lead point is often
found, it is rather uncommon to find a cause in children. Hyperplasia of
Peyer’s patches may be seen in upto a third cases of idiopathic
intussusception [12]. Index patient also had similar pathology at the
site of intussusception.
Contributors: PR: pathology protocol
presentation, writing of manuscript, review of literature; DL: clinical
protocol presentation, writing of manuscript, review of literature; AKJ:
clinical protocol presentation, writing of manuscript, editing of
manuscript, review of literature, patient management; KG: pathology
protocol presentation, review of literature, editing and final approval
of manuscript; AR: laboratory investigations, review of literature,
editing and final approval of manuscript.
Discussants: Clinical discussant and Pediatrician
1: Devika Laishram; Senior resident of treating unit: Ankur Kumar Jindal;
Pathologist 1: Poojitha Reddy; Pathologist 2: Kirti Gupta; Pediatrician
2: Amit Rawat; Pediatrician 3: Surjit Singh.
Funding: None; Competing interest: None
stated.
References
1. Marciano BE, Huang C-Y, Joshi G, Rezaei N,
Carvalho BC, Allwood Z, et al. BCG vaccination in patients with
severe combined immunodeficiency: Complications, risks, and vaccination
policies. J Allergy Clin Immunol. 2014;133:1134-41.
2. Venaille A, Viola S, Peycelon M, Grand d’Esnon A,
Fasola S, Audry G, et al. [Recurrent small-bowel intussusceptions
revealing a disseminated BCG infection related to severe combined
immunodeficiency]. Arch Pediatr. 2014;21: 879-81.
3. Kwan A, Abraham RS, Currier R, Brower A,
Andruszewski K, Abbott JK, et al. Newborn screening for severe
combined immunodeficiency in 11 screening programs in the United States.
JAMA. 2014;312:729-38.
4. Rivers L, Gaspar HB. Severe combined
immunodeficiency: recent developments and guidance on clinical
management. Arch Dis Child. 2015;100:667-72.
5. Chinn IK, Shearer WT. Severe combined
immunodeficiency Disorders. Immunol Allergy Clin North Am.
2015;35:671-94.
6. Cirillo E, Giardino G, Gallo V, D’Assante R,
Grasso F, Romano R, et al. Severe combined immunodeficiency—an
update. Ann N Y Acad Sci. 2015;1356:90-106.
7. Szczawińska-Popłonyk A, Jończyk-Potoczna K,
Ossowska L, Bręborowicz A, Bartkowska-Śniatkowska A, Wachowiak J.
Cytomegalovirus pneumonia as the first manifestation of severe combined
immunodeficiency. Cent-Eur J Immunol. 2014;39:392-5.
8. Notarangelo L. Primary immunodeficiencies. In:
Male D, Brostoff J, Roth DB, Roitt IM, editors. Immunology. 8th ed.
Amsterdam: Elsevier; 2013. p. 263-4.
9. Nickels AS, Boyce T, Joshi A, Hagan J. Absence of
the thymic shadow in a neonate suspected of primary immunodeficiency:
Not a straightforward clinical sign of immunodeficiency. J Pediatr.
2015;166:203.
10. Gupta K, Rawat A, Agrawal P, Jindal A, Nada R,
Saikia B, et al. Infectious and non-infectious complications in
primary immunodeficiency disorders: An autopsy study from North India. J
Clin Pathol. 2018;71:425-35.
11. Nezelof C. Thymic pathology in primary and
secondary immunodeficiencies. Histopathology. 1992;21:499-511.
12. Pang LC. Intussusception revisited:
Clinicopathologic analysis of 261 cases, with emphasis on pathogenesis.
South Med J. 1989;82:215-28.
|
|
|
 |
|

