|
|
|
Indian Pediatr 2018;55:671-674 |
 |
Encephalitic
presentation of Neonatal Chikungunya: A Case Series
|
|
Arti Maria 1,
Nagaratana Vallamkonda1,
Amlin Shukla1,
Aditya Bhatt1 and
Namrita Sachdev2
From Departments of 1Neonatology and
2Radiology, Postgraduate Institute of Medical Education and
Research and Dr RML Hospital, New Delhi, India.
Correspondence to: Dr Arti Maria, Professor and Head,
Department of Neonatology, PGIMER and Dr RML Hospital, New Delhi 110
001, India.
Received: August 06, 2017;
Initial Review: January 01, 2018;
Accepted: May 28, 2018.
|
Objective: To describe clinical features and early neurological
outcomes in neonatal Chikungunya. Methods: Clinical, pathological
and radiological details of neonates with acute encephalitic features
and typical rash, later diagnosed as Chikungunya, are presented.
Neurodevelopmental evaluation and imaging was done at discharge/three
months. Results: Abnormal neurological examination with fever was
typical presentation in all 13 babies with/without seizures/peri-oral
rashes; 12 had persistent neurological abnormalities at discharge. A
follow-up at three months revealed continued neurodevelopmental
deficits. Neuroimaging abnormalities were seen in eight out of ten
cases. Conclusions: Perinatal Chikungunya should be considered in
neonates presenting within first week with fever, encephalopathy and
perioral rashes with/without seizures with history of maternal
Chikungunya within last week before delivery.
Keywords: Acute encephalitis syndrome; Meningoencephalitis;
Neonate; Rash.
|
|
C
hikungunya infection has seen a re-emergence in
India [1]. Typically described as a disease of adult and pediatric
population presenting with fever, arthralgia, arthritis, rash and
constitutional symptoms [2]. It is rarely considered as a diagnosis in
neonates, with little knowledge about natural history and clinical
features. Neurotropism of Chikungunya is under-reported even in adults
and children, and much rarely been described in neonates [3]. We
describe clinical and laboratory features of neonates presenting with
acute encephalitis syndrome and later diagnosed as Chikungunya
infection.
Methods
The medical record review was conducted in the
neonatal intensive care unit of a tertiary-care center in Delhi. Babies
who presented with neonatal sepsis like features (fever, lethargy, poor
suck, feed intolerance, respiratory distress, jaundice, skin rash,
seizures, shock, DIC etc.) but not supported by laboratory workup
for bacterial sepsis/meningitis were worked up for Chikungunya in view
of outbreak in the city (especially, in those with history of maternal
fever). NIV CHIK IgM Capture Enzyme-linked immunosorbent assay (ELISA)
(version 3, 4) was done in serum samples after obtaining consent from
parents. CSF Reverse Transcriptase Polymerase Chain reaction (RT-PCR)
was done from a reference laboratory. A large number of cases in a short
period prompted us to plan their detailed neuro-developmental follow-up,
and information about clinical features and laboratory investigations
was compiled retrospectively from case records. Neurosonogram and MRI
brain were done. Neurological examination was done using Hammersmith
neonatal examination tool at discharge/at three months of age.
A congenital Chickungunya case was defined as a baby
born to mother with high grade fever within seven days before delivery
with IgM seropositivity or CSF-positivity at time of neonatal diagnosis,
or a symptomatic baby in first seven days of life having a positive IgM
ELISA/RT-PCR in serum/CSF and negative bacterio-logical cultures. Any
seropositivity or CSF-positivity found in a symptomatic baby not
associated with maternal infection was defined as acquired Chikungunya.
Results
Ninety nine babies were admitted between August and
November, 2016, out of which 13 (11 males) were confirmed as Chikungunya.
10 cases were congenital, two acquired. One baby was adopted and
maternal clinical and laboratory status were not known. Encephalopathy
was present in all with duration varying from 10-15 days; 11 presented
with fever and six presented with seizures (Table I). Ten
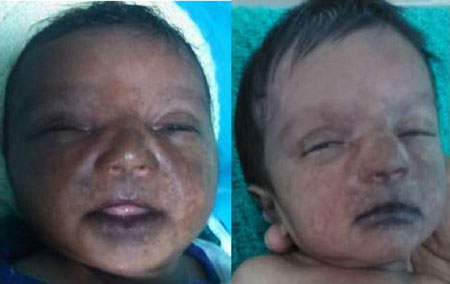
cases had a characteristic acrofacial hyperpigmentation beginning three
to four days after fever in peri-oral region leading to characteristic
‘brownie-nose pigmentation’ (Fig. 1), later spreading to
trunk and limbs in patchy fashion. Shock was present in two cases but
was successfully treated and no death occurred. Hammersmith neonatal
examination showed hypotonia in 12 out of 13 infants at discharge;
visual and auditory development was normal in all 13 babies.
TABLE I Clinical and Laboratory Features of Neonates with Chikunguniya (N=13) [4]
|
Clinical features |
n |
|
Symptoms |
|
|
Lethargy |
13
|
|
Fever |
11
|
|
Feed refusal |
13
|
|
Convulsions |
6
|
|
Hyperpigmentation |
10
|
|
Hypotonia |
13
|
|
Investigations
|
|
|
Leucopenia <6000/cumm |
3 |
|
Thrombocytopenia <50,000/µL |
12
|
|
CRP >1 mg% |
6
|
|
Positive serum IgM (cases) |
13 |
|
Positive serum IgM (Mother) |
10
|
|
USG Cranium at discharge |
13 normal
|
|
USG Cranium at 3 months, n=2 |
2 abnormal
|
|
MRI at 3 months, n=10 |
8 abnormal
|
|
OAE, n=12 |
12 normal |
|
Cerebrospinal fluid Parameters |
|
|
Hypoglycorrhachia (<45 mg%) |
9 |
|
Increased protein (>80 mg%) |
9 |
|
Pleocytosis (>15 cells/mm3) |
2 |
|
Sterile culture |
13 |
|
CRP=C-reactive protein, USG=ultrasonography; MRI=Magnetic
resonance imaging; OAE=Otoacoustic emission. |
 |
|
Fig. 1 Acro-facial cutaneous
hyperpigmentation.
|
Neuro-developmental assessment at three months
demonstrated four children with delayed milestones, out of which two
cases each had hypotonia and hypertonia. Seven cases had normal tone and
achieved normal milestones. Hyperpigmentation disappeared in all babies.
One baby showed additional flexion deformity in bilateral thumbs and
right middle finger at one month, which resolved by three months with
the help of physiotherapy. Two babies were lost to follow-up.
CSF-RT-PCR could only be done in one neonate which
was positive. CSF culture was sterile in all cases with cytology typical
of viral meningoencephalitis. CSF was completely normal in one baby.
Thrombocytopenia was present in 12 babies but none had clinical bleeding
(Table I).
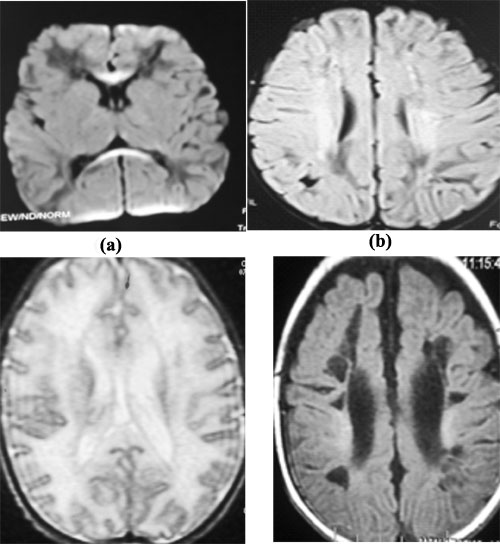
MRI brain had evidence of white matter
hyperintensities on T2 and FLAIR images involving frontal and parietal
lobes in bilateral peri-ventricular and subcortical region with evidence
of diffusion restriction in rostrum and splenium of corpus callosum in
three patients. No post-contrast enhancement/hemorrhage or infra-tentorial/cerebellar
involvement was seen.
Follow up MRI scan after three months revealed cystic
encephalomalacia and ventricular dilatation in two cases, and diffuse
cerebral atrophy in one (Fig. 2). Oto-acoustic emissions
(OAE) screening was normal in 12 babies and abnormal in one at
discharge, which normalized at three months.
 |
| (c) |
(d) |
| |
Discussion
A major finding in this study was that neonatal
Chikungunya was quite common during the outbreak. Congenital infection
was more frequent than acquired. A striking clinical feature was
neurotropism, which was evident in all cases in form of refusal to feed,
lethargy and seizures. Another common clinical finding was
hyperpigmentation following a typical pattern. Laboratory features were
conspicuous by thrombo-cytopenia and positive serum IgM Chikungunya.
Initial USG cranium was normal in all cases but follow-up MRI scans were
abnormal in majority, along with developmental delay in these cases.
The study had a few limitations as information was
collected retrospectively and all cases were from referral unit which
could have resulted in bias. No statistical tests could be performed due
to small number of patients.
In our study, more male infants were admitted with
the infection, similar to another Indian study [5]. Most cases were
prenatally acquired similar to a data from the French Reunion islands
[6]. In contract to the predominant encephalopathic presentation in this
study, a Latin American study [7] showed only a 7.1% incidence of
meningoencephalitis in newborns. However, review of their study shows
that 98-100% babies presented with irritability and refusal to feed,
which may suggest presence of encephalopathy. The cohort in this study
was from intramural deliveries while ours were referred babies.
A higher incidence of encephalitis in neonates with
Chikungunya could be attributed to greater viral replication and delayed
clearance in infants [8]. Ineffective interferon-1 activation has been
demonstrated in mice to be associated with severe disease. Neurological
spread occurs through areas in brain poorly protected by blood brain
barrier [9]. The plausible mechanisms of neuronal damage are: invasion
of choroid plexus and leptomeninges leading to defective neuronal
migration. Another possibility could be microglial activation [10]. The
study on mice also demonstrated that the vertical transmission of the
virus is Peri-partum and not ante-partum [9].
Similar radiologic and developmental findings were
noticed in a cohort study from Reunion Island [10]. Peri-oral hyper
pigmentation fever, leukopenia and mild thrombocytopenia were a
predominant feature in our study, similar to another study [5],
post-inflammatory hyperpigmentation could be due to virus-triggered
increased intra-epidermal dispersion/retention of melanin [11]. Fixed
flexion deformity in bilateral thumb reported in one of our babies is
similar to another case-report [12].
Our study shows that Chikungunya should be considered
as a differential diagnosis in neonates presenting with fever, typical
hyperpigmentation and encephalopathy especially during outbreaks. Cases
with perinatal infection are prone to developmental delay and require
long term neuro-developmental follow-up.
Acknowledgements: Dr Anthony Costello for editing
the manuscript and Dr Rakesh Dey for helping in data collection.
Contributors: AM: conceptualized and
designed the study and revised it critically; NV: acquisition of
data, initial analysis, and reviewed and revised manuscript; AS:
analysis and interpreted blood and CSF findings, and critically reviewed
the manuscript; AB: drafted the initial manuscript, and reviewed
the manuscript; NS: Analysis and interpretation of Radiological
findings and reviewed the manuscript. All authors approved the final
manuscript and agree to be accountable for all aspects of work.
Funding: None; Competing interests: None
stated.
|
What This Study Adds?
•
Neonatal Chikungunya should be
considered in neonates presenting with fever, encephalopathy and
cutaneous hyperpigmentation with/without seizures.
•
Confirmed cases need a detailed neurodevelopmental
follow-up.
|
References
1. Directorate General of Health Services, Ministry
of Health and Family Welfare, Government of India. National Vector Borne
Disease Control Programme. Available from:
http://nvbdcp.gov.in/chik-cd.html. Accessed January 28, 2018.
2. Da Cunha RV, Trinta KS. Chikungunya virus:
Clinical aspects and treatment - A review. Mem Inst Oswaldo Cruz.
2017;112:523-31.
3. Chandak NH, Kashyap RS, Kabra D, Karandikar P,
Saha SS, Morey SH, et al. Neurological complications of
Chikungunya virus infection. Neurol India. 2009;57:177-80.
4. MacDonald MG, Seshia MMK. Avery’s Neonatology:
Pathophysiology and Management of the Newborn. 7th ed; 2015. p.
967.
5. Valamparampil JJ, Chirakkarot S, Letha S,
Jayakumar C, Gopinathan KM. Clinical profile of Chikungunya in infants.
Indian J Pediatr. 2009;76:151-5.
6. Lenglet Y, Barau G, Robillard PY, Randrianaivo H,
Michault A, Bouveret A, et al. Chikungunya infection in
pregnancy: Evidence for intrauterine infection in pregnant women and
vertical transmission in the parturient. Survey of the Reunion Island
outbreak. J Gynecol Obstet Biol Reprod (Paris). 2006;35:578-83.
7. Torres JR, Falleiros-Arlant LH, Duenas L,
Pleitez-Navarrete J, Salgado DM, Castillo JB. Congenital and perinatal
complications of chikungunya fever: A Latin American experience. Int J
Infect Dis. 2016 Oct;51:85-8.
8. Mohanty I, Dash M, Sahu S, Narasimham MV, Panda P,
Padhi S. Seroprevalence of chikungunya in Southern Odisha. J Family Med
Prim Care. 2013;2:33-6.
9. Couderc T, Chretien F, Schilte C, Disson O,
Brigitte M, Guivel-Benhassine F, et al. A mouse model for
Chikungunya: Young age and inefficient type-I interferon signaling are
risk factors for severe disease. PLoS Pathog. 2008;4:e29.
10. Gerardin P, Samperiz S, Ramful D, Boumahni B,
Bintner M, Alessandri JL, et al. Neurocognitive outcome of
children exposed to perinatal mother-to-child Chikungunya virus
infection: The CHIMERE cohort study on Reunion Island. PLoS Negl Trop
Dis. 2014;8:e2996.
11. Seetharam KA, Sridevi K, Vidyasagar P. Cutaneous
manifestations of chikungunya fever. Indian Pediatr. 2012;49:51-3.
12. Gopakumar H, Ramachandran S. Congenital
chikungunya. J Clin Neonatol. 2012;1:155-6.
|
|
|
 |
|

