|
|
|
Indian Pediatr 2017;54: 641-643 |
 |
Comparison of
Transcutaneous Bilirubin Measurement With Total Serum Bilirubin
Levels in Preterm Neonates Receiving Phototherapy
|
|
Amruta Pendse, Bonny Jasani, Ruchi Nanavati and
Nandkishor Kabra
From Department of Neonatology, KEM Hospital, Parel,
Mumbai, India.
Correspondence to: Dr Amruta Pendse, Suite 1, 611
Murray Street, West Perth, Western Australia, 6005, [email protected]
Received: October 14, 2016;
Initial Review: February 08, 2017;
Accepted: June 03, 2017
|
|
Objective: To compare transcutaneous bilirubin
with total serum bilirubin in preterm neonates after initiation of
phototherapy. Methods: Jaundice was assessed in 30 preterm
neonates with transcutaneous bilirubin and total serum bilirubin before
initiation of phototherapy and at 12 hr after initiation of
phototherapy. A photo-occlusive patch was applied over the sternum.
Results: Transcutaneous bilirubin has a good correlation with total
serum bilirubin after initiation of phototherapy. (r=0.918, P<0.001).
Transcutaneous bilirubin at 28-32 weeks of gestation (r = 0.97) was
better correlated with total serum bilirubin than those at 32-37 weeks
(r =0.88). The correlation was better for neonates <72 hours old (r =
0.96) than those >72 hours of age (r = 0.82). Conclusion:
Transcutaneous bilirubin correlates significantly with total serum
bilirubin at the patched sternal site after initiation of phototherapy
in preterm neonates.
Keywords: Assessment, Diagnosis, Hyperbilirubinemia, Jaundice.
|
|
|
|
P
reterm neonates are susceptible to higher risk of
kernicterus at lower bilirubin values. Transcutaneous bilirubin (TcB)
testing has the advantages of instantaneous results and avoidance of
repeated blood sampling [1]. However, its use after the initiation of
phototherapy (PT) has not been reliably studied in preterm neonates
[2-4]. We planned to compare TcB with total serum bilirubin (TSB) in
preterm neonates after initiation of PT over a patched sternal area.
Methods
The study was conducted in a level III neonatal
intensive care unit from September 2014 to February 2015. The study
protocol was approved by the institutional ethics committee. Written
informed consent was obtained from either of the parents or guardian
prior to enrollment in the study.
Preterm neonates >28 and <37 weeks gestation having
clinically detectable jaundice were included and neonates with
conjugated hyperbilirubinemia, evidence of hemolysis or poor perfusion
were excluded.
TSB was estimated using acid diazo method (Vanden
Bergh reaction). Simultaneously, the TcB was measured on sternum using
Drager jaundice meter JM 105. Average of three consecutive readings was
recorded in mg/dL. The device was calibrated before usage according to
the manufacturer’s recommendations [5].
PT (compact fluorescent light or light emitting diode
units with an irradiance of 20-30 µW/cm2/nm) was instituted if the TSB
fulfilled the criteria as per sliding scale for preterm neonates [6]. A
patch of skin over the sternum was shielded using a maxicor electrode
covered with aluminium foil [7]. A repeat TSB and TcB assessment was
done 12 hours after the initiation of PT on the shielded skin area. The
TSB and TCB were recorded within 15 minutes of each other. No additional
blood investigations were done for the purpose of the study. The skin
integrity was assessed with the Neonatal skin condition score (NSCS)
before and after the application of the skin patch [8].
The primary outcome was comparison of TcB with TSB in
preterm neonates after initiation of phototherapy. Secondary outcome was
to compare TcB with TSB after initiation of phototherapy according to
gestational age (28-32 vs 32-37 weeks) and postnatal age (<72
vs >72 hours).
Statistical analysis: Sample size was calculated
by using formula for correlation coefficient using z transformation.
From previous studies the correlation coefficient between TcB and TSB
measurement varies between r = 0.5 to 0.9. Assuming alpha error
of 0.05, beta error of 0.2 and r value of 0.5, estimated sample size was
29. A scatter plot was used to depict the relationship between TcB and
TSB. Correlation coefficients were calculated using Pearson correlation
(parametric test) or Spearman rank correlation (nonparametric test). A
P value of <0.05 was considered as statistically significant.
Bland-Altman analysis was used to visualize the agreement between TSB
and TCB.
Results
The study included 30 (12, 28-32 wks; 18, 32-37 wks)
preterm neonates. The baseline characteristics are shown in Table
1. TcB estimated at sternum correlated significantly with TSB
prior to initiation of phototherapy (r=0.903, P<0.001) and after
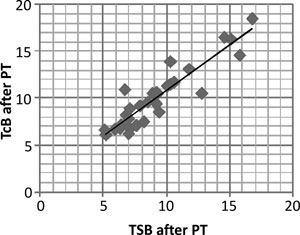
phototherapy over the patched sternal area (r=0.918, P<0.001) (Fig.
1a). The mean difference between TcB and TSB after initiation
of PT was 0.87 mg/dL. TcB overestimated TSB in majority of the readings
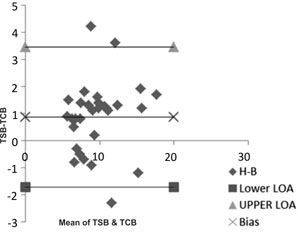
(76.7%) more so for higher levels of TSB (>10 mg/dL). Using the Bland
Altman analysis (Fig. 1b), 90% of the data points
were in the 95% confidence interval which are the limits of agreement.
By regression analysis, the mean differences between TcB and TSB were
not statistically significant (P=0.512).
TABLE I Baseline Characteristics of Study Population
|
Characteristic |
|
|
*Birth weight (g) |
1680 (633.6) |
|
*Gestational Age (wks) |
32.9 (2.6) |
|
#APGAR at 1 min |
8 (7-9) |
|
#APGAR at 5 min |
9 (8-9) |
|
Cesarean Section, n (%) |
14 (46.7) |
|
Small for gestation at age, n (%) |
9 (30) |
|
Male, n (%) |
13 (43.3) |
|
*Age at estimation of bilirubin before PT (h) |
70.4 (24.9) |
|
Average time (h) of taking TcB after initiation of PT |
12.58 |
|
*TSB before PT (mg/dL) |
12.05 (3.49) |
|
*TcB before PT (mg/dL) |
13.03 (3.83) |
(a) |
(b) |
|
Fig. 1 (a) Scatter plot depicting
correlation; (b) Bland altman analysis.
|
TcB in babies 28-32 weeks of gestation (r=0.97;
P<0.001) were better correlated with TSB than in 32-37 weeks (r=0.88;
P<0.001). The correlation coefficient was better for neonates <72
hours (r=0.96; P<0.001) than those >72 hours of age (r=0.82;
P<0.001). None of the neonates had evidence of loss of skin
integrity as assessed by NSCS.
Discussion
This study showed a positive correlation between TSB
and patched TcB in preterm neonates after starting PT.
Previous studies have demonstrated a good agreement
between TSB and patched TcB during PT in preterm neonates [7,9,10].
However, in a study by Jangaard, et al. [11], TcB measurement
during PT was not found to be as sensitive in preterm compared to term
neonates.
The difference noted between 32-37 week and 28-32
weeks gestation groups could be explained by the skin immaturity of very
preterm neonates. There are no studies till date which have evaluated
this difference. The correlation coefficient was better for neonates <72
hours than those >72 hours of age. We hypothesize that as the skin
pigmentation increases with age, correlation begins to decline.
Limitations of the present study are lack of
comparison with other sites like forehead and inter-scapular area [12].
Also, serial TcB measurements from the patched site after starting PT
could have been a better guide to evaluate the trends in correlation
during the course of PT.
This study has major implications for developing
countries where the rate of prematurity is high, necessitating prolonged
NICU admissions, phlebotomy losses and unavailability of micro-methods
for bilirubin estimation in most laboratories.
Acknowledgement: Dean, Seth GS Medical College
and KEM Hospital, Mumbai for permission to publish the manuscript.
Contributors: All authors have written, designed
and approved the study. Funding: None; Competing interest:
None stated.
References
1. Maisels MJ, Ostrea EM Jr, Touch S, Clune SE,
Cepeda E, Kring E, et al, Evaluation of a new transcutaneous
bilirubinometer. Pediatrics. 2004; 113:1628-35.
2. Ozkan H, Oren H, Duman N, Duman M. Dermal
bilirubin kinetics during phototherapy in term neonates. Acta Pediatr.
2003;92:577-81.
3. Nagar G, Vandermeer B, Campbell S, Kumar M.
Reliability of transcutaneous bilirubin devices in preterm infants: a
systematic review. Pediatrics. 2013;132:871-81.
4. Juster-Reicher A, Flidel-Rimon O, Rozin I,
Shinwell ES. Correlation of transcutaneous bilirubinometry (TcB) and
total serum bilirubin (TsB) levels after phototherapy. J Matern Fetal
Neonatal Med. 2014;30:1-3.
5. Jaundice Meter JM-105 instruction manual. 2014.
Drägerwerk AG and Co. KGaA. Ref Type: Generic.
6. Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK.
An approach to the management of hyperbilirubinemia in the preterm
infant less than 35 weeks of gestation. J Perinatol. 2012;32:660-4.
7. Povaluk P, Shwetz EA, Kliemann R. Comparative
study between plasma and transcutaneous bilirubin measure-ments in
newborns. Rev Paul Pediatr. 2011;29:6-12.
8. Lund CH, Osborne JW. Validity and reliability of
neonatal skin condition score. J Obstet Gynaecol Neonatal Nurs.
2004;33:320-7.
9. Zecca E, Barone G, De Luca D, Marra R, Tiberi E,
Romagnoli C. Skin bilirubin measurement during phototherapy in preterm
and term newborn infants. Early Hum Dev. 2009;85:537-40.
10. Nanjundaswamy S, Petrova A, Mehta R, Hegyi T.
Transcutaneous bilirubinometry in preterm infants receiving
phototherapy. Am J Perinatol. 2005;22:127-31.
11. Jangaard KA, Curtis H, Goldbloom R. Estimation of
bilirubin using BiliChek trade mark, a transcutaneous bilirubin
measurement device: Effects of gestational age and use of phototherapy.
Paediatr Child Health. 2006;11:79-83.
12. Conceição CM, Dornaus MF, Portella MA, Deutsch
AD, Rebello CM. Influence of assessment site in measuring transcutaneous
bilirubin. Einstein (Sao Paulo). 2014;12:11-5.
|
|
|
 |
|

