|
|
|
Indian Pediatr 2015;52:
691-693 |
 |
Short-term Ibuprofen Treatment and Pulmonary
Function in Children with Asthma
|
|
Yu-Mao Su, Che-Sheng Huang and Kong-Sang Wan
From Department of Pediatrics, Taipei City Hospital-Renai
Branch, Taiwan
Correspondence to: Dr Kong-Sang Wan, Department of
Pediatrics, Taipei City Hospital-Renai Branch, No.10, Sec.4, Renai Road,
Da An District, Taipei City 10629, Taiwan.
Email: gwan1998@gmail.com
Received: July 21, 2014;
Initial review: September 08, 2014;
Accepted: April 29, 2015.
|
Objectives: To investigate the
association between ibuprofen use and pulmonary function in children
with Asthma.
Methods: Ninety 9- to 10-year-old children
were classified into 3 groups: Study group, mild to moderate
stable asthmatic children with self-reported aspirin allergy and no
history of anaphylaxis; Allergy control group: atopic children
(allergic rhinitis/atopic dermatitis); Healthy control group:
non-atopic healthy children. None of the participants in the atopic and
healthy control groups had a history of aspirin allergy. All received
ibuprofen 4 times a day for 3 consecutive days. Forced expiratory volume
in the first second (FeV1) and fractional exhaled nitric oxide (FeNO)
measurements were performed before and after ingestion of ibuprofen
daily for 3 days.
Results: In the study group, a decrease in
FeV1 and increase in FeNO levels were observed after taking ibuprofen
for 2 days. The atopic control group showed only an increase in FeNO but
not FEV1. In the healthy control group, both FeV1 and FeNO were
unchanged from baseline.
Conclusions: The results showed that
cross-reactive non-steroidal anti-inflammatory drug hypersensitivity may
exist between ibuprofen and aspirin. This raises the possibility that
asthma exacerbation could be mediated by ibuprofen ingestion.
Keywords: Asthma, Exhaled nitric oxide, FeV 1,
Ibuprofen, NSAID.
|
|
Non-steroidal anti-inflammatory drugs (NSAIDs) are
one of the most commonly involved groups of medicines in
hypersensitivity drug reactions. About 76% of patients who are
hypersensitive to NSAIDs have cross-intolerance, and atopy can be a
predisposing factor in patients with cross-intolerance [1,2]. Aspirin
intolerance in asthmatic patients has been reported to be underdiagnosed
[3]. In those who are allergic to NSAIDs, acetylsalicylic acid (18.2%)
and ibuprofen (18.2%) are the most frequently identified drugs [4]. In
patients with hypersensitivity reactions to NSAIDs, 76% have
cross-intolerance and 24% are selective responders. The most important
drugs involved in cross-intolerance are propionic acid derivatives, in
most cases ibuprofen, and in sustained-release pyrazolones. In
cross-intolerance, the most frequently reported clinical entities are
urticaria and angioedema; however, the airway can also be involved [1].
It has been suggested that the possibility of ibuprofen-induced
bronchospasms should be considered before administering ibuprofen to
children with asthma [5]. Moreover, this asthmatic reaction is
dose-dependent and can occur with sub-therapeutic doses [6]. We,
therefore, investigated the effect of short-term ibuprofen treatment on
pulmonary function in asthmatic children.
Methods
Ninety 9- to 10-year-old children (49 males) were
enrolled and divided into three groups of 30 children each. The Study
group comprised of children with mild to moderate asthma as classified
by the GINA guidelines and a self-reported history of aspirin-allergy.
The Allergic control group had children with allergic rhinitis or atopic
dermatitis, and the Healthy control group included healthy children
attending our hospital for vaccination. The children in the allergy and
healthy control groups had no history of aspirin-allergy. Forced
expiratory volume in the first second (FeV 1)
and Fractional exhaled nitric oxide (FeNO) measurements (NIOX MINO
system, Phadia) were performed for each participant every morning before
breakfast for 3 consecutive days. The participants took ibuprofen 2.5
mg/kg 4 times a day. We defined bronchospasm as a
³20% decrease from
baseline in FeV1, and
ibuprofen-sensitivity as bronchospasm following administration of
ibuprofen. Asthma exacerbation was defined as an increase in FeNO level
from baseline (20 ppb) with increased coughing frequency and chest
tightness clinically, increased use of inhaled corticosteroids (ICS) or
short-acting beta-agonists, and a decrease in PaO2
of less than 90%. Children taking systemic steroids within one week of
the initiation of the study were excluded. This study was approved by
the Institutional Review Board of our hospital, and all of the
participants’ parents or guardians provided written informed consent.
The Statistical Package for Social Sciences software
version 12 for Windows (SPSS Inc., Chicago, IL, USA) was used for all
statistical analyses. Statistical significance was set at P<0.05.
Results
After the children had received the intervention for
two days, a worsening of pulmonary function and asthma exacerbation was
seen in a significant number of children in the study group (Table
I). The allergy control group only showed an increased FeNO level
but no change in FeV 1. In
the healthy control group, both PFT and FeNO were maintained at the
baseline level (Fig. 1).
TABLE I Clinical and Laboratory Parameters in the Study Population After Taking Ibuprofen for Two Days
|
Study group |
Allergic control group |
Healthy control group |
P value |
|
(n=30) |
(n=30) |
(n=30) |
|
|
FEV1 (% of prediction) |
80.97 ± 5.35 |
95.42 ± 4.79 |
97.10 ± 1.12 |
<0.001 |
|
FeNO (ppb) |
31.47 ± 4.78 |
28.67 ± 3.85 |
9.50 ± 1.57 |
<0.001 |
|
PaO2 (mm Hg) |
85.23 ± 2.40 |
92.60 ± 3.45 |
97.17 ± 2.09 |
<0.001 |
|
*Increased cough and/or chest tightness |
30 (100) |
6 (20.0) |
0 |
<0.001 |
|
*Increased ICS dose and/or SABA use |
30 (100) |
5 (16.7) |
0 |
<0.001 |
|
FEV1: Forced expiratory volume in the first second; FeNO:
Fractional exhaled Nitric Oxide; PaO2: Partial pressure of
Oxygen in arterial blood; ICS: Inhaled corticosteroid; SABA:
short acting beta-agonist. Values in mean ± SD or * No. (%). |
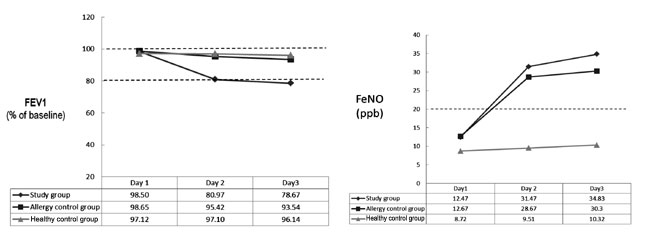 |
|
Fig. 1 (a) The FEV1 changes in the
study group after treatment of ibuprofen for 3 consecutive days;
(b) The FeNO changes in the study group after treatment with
ibuprofen for 3 consecutive days.
|
Discussion
As ibuprofen has cross-intolerance with aspirin, the
children with mild to moderate asthma in this study had an increased
frequency of coughing with or without short of breath, and required
double the dose of ICS or the use of short acting beta-agonists to ease
the exacerbation of asthma symptoms. Patients with aspirin-exacerbated
respiratory diseases typically experience severe bronchoconstriction
and/or rhinoconjunctival reactions to aspirin and other NSAIDs, even
those which they have not encountered previously [7].
Aspirin-intolerance, as determined in broncho-provocation studies, may
be apparent in 5% of asthmatic children [8]. Palmer reported that
NSAID-exacerbated asthma may occur in up to 2% of asthmatic children
[6].
Kanabar, et al. [9] reported that ibuprofen
use was associated with a low relative risk for hospitalization (0.63)
and outpatient visits (0.56) for asthma compared with acetaminophen. In
addition, Kidon, et al. [10] also reported that ibuprofen at
antipyretic doses may cause acute respiratory problems in only a very
small number of mild to moderate asthmatic children. Furthermore, a
cross-reactive hypersensitivity response to NSAIDs was reported in 45%
of young Asian atopic children through their history, and in 25% through
diagnostic challenge [11].
Of the patients with hypersensitivity reactions to NSAIDs, 76% had
cross-intolerance and 24% were selective responders. The most important
drugs involved in cross-intolerance are propionic acid derivatives, in
most cases ibuprofen, and in selective responders, pyrazolones. In
cross-intolerance, the most frequent clinical entities are urticaria and
angioedema, and to a lesser extent airway involvement [1]. Jenkins,
et al. [12] reported that cross-intolerance to doses of NSAIDs was
present in most patients with aspirin-induced asthma (ibuprofen, 98%;
naproxen, 100%; and diclofenac, 93%). On the other hand, beneficial
effects of ibuprofen have been reported in cystic fibrosis [13]. In
addition, due to the inflammatory pathogenesis of asthma, the
anti-inflammatory effect of ibuprofen may possibly reduce morbidity in
children with asthma.
There are several limitations to this study. First,
the number of enrolled subjects was limited. Second, other NSAIDs such
as diclofenac sodium and naproxen were not evaluated for
cross-intolerance comparative studies. Third, both sub-clinical and high
doses of ibuprofen were not included.
Ibuprofen is used extensively among children as an
analgesic and antipyretic agent. However, whether children with asthma
or are at risk of developing asthma should avoid the use of ibuprofen
still remains to be elucidated [15]. In Taiwan, ibuprofen is commonly
used over-the counter and in hospitals due to the advantage of a less
frequent dose and a longer duration of action. In clinical practice, the
possibility of ibuprofen-induced bronchospasm should be considered
before administering ibuprofen to children with asthma.
|
What This Study Adds?
• Exposure to ibuprofen worsens the pulmonary
functions and exacerbates asthmatic symptoms in children with
asthma having aspirin-allergy.
|
References
1. Dona I, Blanca-Lopez N, Cornejo-Garia JA, Torres
MJ, Laguna JJ, Fernández J, et al. Characteristics of subjects
experiencing hypersensitivity to non-steroidal anti-inflammatory drugs:
patterns of response. Clin Exp Allergy. 2011;41:86-95.
2. Szczeklik A, Stevenson DD. Aspirin-induced asthma:
advances in pathogenesis and management. J Allergy Clin Immunol.
1999;104:5-13.
3. Szczeklik A, Nizankowska E. Clinical features and
diag-nosis of aspirin induced asthma. Thorax. 2000;55:S42-4.
4. Gomes E, Cardoso MF, Praca F, Gomes L, Mariño E, Demoly
P. Self-reported drug allergy in general adult Portuguese population.
Clin Exp Allergy. 2004;34:1597-601.
5. Debley JS, Carter ER, Gibson RL, Rosenfeld
M, Redding GJ. The prevalence of ibuprofen-sensitive asthma in children:
a randomized controlled bronchoprovocation challenge study. J Pediatr.
2005;147:233-8.
6. Palmer GM. A teenager with severe asthma
exacerbation following ibuprofen. Anaesth Intensive Care. 2005;33:261-5.
7. Stevenson DD. Aspirin sensitivity and
desensitization for asthma and sinusitis. Curr Allergy Asthma Rep.
2009;9:155-63.
8. Jenkins C, Costello J, Hodge L. Systematic review
of prevalence of aspirin induced asthma and its implications for
clinical practice. BMJ. 2004;7437:434-7.
9. Kanabar D, Dale S, Rawat M. A review of ibuprofen
and acetaminophen use in febrile children and the occurrence of
asthma-related symptoms. Clin Ther. 2007;29:2716-23.
10. Kidon MI, Kang LW, Chin CW, Hoon LS, Hugo VB,
et al. Nonsteroidal anti-inflammatory drug hypersensitivity in
preschool children. Allergy Asthma Clin Immunol. 2007;3:114-22.
11. Kidon MI, Kang LW, Chin CW, Hoon LS, See Y, Goh
A, et al. Early presentation with angioedema and urticaria in
cross-reactive hypersensitivity to nonsteroidal anti-inflammatory drugs
among young, Asian, atopic children. Pediatrics. 2005;116:e675-80.
12. Jenkins C, Costello J, Hodge L. Systematic review
of prevalence of aspirin induced asthma and its implications for clinic
practice. BMJ. 2004;328:434.
13. Lands LC, Milmer R, Cantin AM, Manson D, Corey
M. High-dose ibuprofen in cystic fibrosis: Canadian safety and
effectiveness trial. J Pediatr. 2007;151:249-54.
14. Kauffman RE, Lieh-Lai M. Ibufrofen and increased
morbidity in children with asthma: fact or fiction? Paediatr Drugs.
2004;6:267-72.
15. Risser A, Donovan D, Heintzman J, Page T. NSAID prescribing
precautions. Am Fam Physician. 2009;80:1371- 8.
|
|
|
 |
|

