|
|
|
Indian Pediatr 2015;52:
687-690 |
 |
Long-term Immunogenicity of Single Dose of
Live Attenuated Hepatitis A Vaccine in Indian Children
|
|
Sheila Bhave, Amita Sapru, Ashish Bavdekar, *Vaibhavi
Kapatkar and *Amey Mane
From Department of Pediatrics, KEM Hospital Research
Centre, Pune; and *Department of Medical Affairs, Wockhardt Limited,
Mumbai; India.
Correspondence to: Dr Sheila Bhave, Department
of Pediatrics, KEM Hospital Research Centre,
Rasta Peth, Pune 411 011, India.
Email: kemhrc@vsnl.net
Received: December 28, 2014;
Initial review: January 27, 2015;
Accepted: May 29, 2015.
|
Objectives: To assess immunogenicity of a single
dose of live attenuated hepatitis A vaccine in Indian children, ten
years after immunization.
Methods: Of 143 children vaccinated in 2004, 121
children were evaluated in 2014, clinically and for anti-HAV antibodies.
Results: 13 children were early vaccine failures
who received two doses of HAV vaccine subsequently. 106 (98%) of 108
remaining children had seroprotective levels with a geometric mean titer
of 100.5 mIU/mL. On analysis of all 121 children, the immunogenicity was
87.6%.
Conclusion: Single dose of live attenuated
hepatitis A vaccine provides long-term immunity in Indian children.
Keywords: Immunization, Prevention, Protection, Hepatitis A
vaccine.
|
|
Hepatitis A (HAV) vaccine is now recommended by
the World Health Organization (WHO) and Indian Academy of Pediatrics
(IAP) in routine immunization of children, aged one year or above [1-3].
Both inactivated and live attenuated vaccines have been approved. The
general recommendation for inactivated vaccine is to use two doses, 6
months apart. WHO and now, IAP, endorse a single dose schedule for live
HAV vaccine [1,3]. How-ever, IAP until recently recommended two doses,
pending long term immunogenicity studies from India [2].
Single dose of live H2 strain HAV vaccines has been
used in China for over 20 years, and shown remarkable safety,
immunogenicity, and long-term protection [4,5]. The first study outside
China to assess the safety and efficacy of single dose of live
attenuated HAV vaccine (Biovac-A) was conducted at our center in Pune,
India in 2004. It documented excellent immunogenicity (95.8%) two months
after vaccination [6,7]. In this report, we present the results of
immunogenicity in the children enrolled at Pune study center (in 2004)
ten years after vaccination with a single dose of live HAV vaccine.
Methods
Children (age 1-12 y) who were enrolled at the start
of the study in 2004 [6], and who participated in regular follow-up and
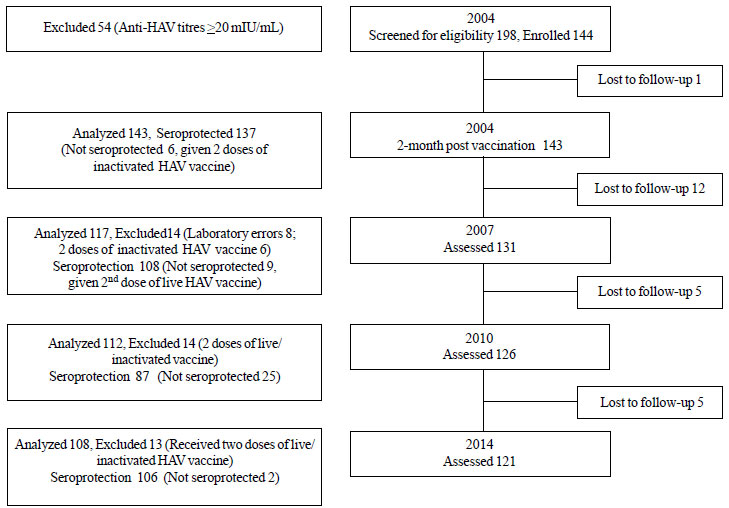
immunogenicity assessments in 2007 and 2010, were re-valuated in 2014 (Fig
1). Institutional ethics committee approval and informed consent
were obtained. Participants were compensated for travel and loss of
wages (where applicable). After routine clinical assessments, blood
samples were collected and sent for estimation of total and IgM anti-HAV
antibody analyses (Cobas
anti-HAV electro-chemiluminescence immuno-assay, ECLIA, Roche
Diagnostics Deutschland GmbH) [8] to an independent accredited
laboratory (SRL Diagnostics, Mumbai, India). Seroprotection rate was
defined as proportion of subjects with total anti-HAV antibody level
³20 mIU/mL.
Geometric mean titers (GMTs) were calculated as per standard method.
 |
|
Fig.1 Flow chart of 10-year study
(2004-2014).
|
Results
Of the original 143 children who received a single
dose of live attenuated HAV vaccine in 2004, 121 subjects reported for
10 year follow-up assessment in 2014. Of these, four who had received
two doses of licensed inactivated HAV vaccine (Havrix, GSK Biologicals)
in 2004 (vaccine failures) and nine who had
received a second dose of live HAV vaccine in 2007 for low titers
(antibody levels <20 mIU/mL), were not evaluated in present analysis (Fig.
1). Therefore, 108 children who received a single dose of the live
vaccine were analyzed.
The clinical examination of the participants did not
reveal any abnormal findings and none had a history of hepatitis-like
illness in the past. In the present analysis, 106 of the 108 included
children had anti-HAV titres ³20
mIU/mL; seroprotection rate of 98.1% (95% CI 93.5%, 99.8%); 10 years
after a single dose of the live attenuated vaccine. Only two
participants had anti-HAV titres <20 mIU/mL (11.5 mIU/mL and 13.5 mIU/mL).
Anti-HAV IgM was negative in all the children studied. The GMT of anti
HAV antibodies of seroprotected children was 100.5 mIU/mL (95% CI 87.4
mIU/mL, 115.4 mIU/mL). On analysis of all 121 children (including 4
‘vaccine failures’ and 9 with low titres at 30 months post-vaccination),
the immunogenicity was 87.6%. The comparison of 10 year immunogenicity
data with previous assessments (2004, 2007 and 2010) is presented in
Fig. 2.
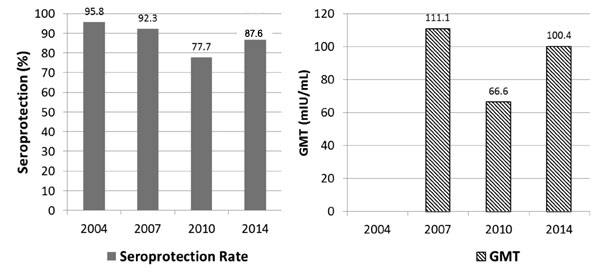
* GMT of 2004 not presented as anti HAV titres >100mIU/mL
were not quantified in 2004.
|
|
Fig. 2 (a) Serial Seroprotection Rates
(2004-2014); (b) Serial GMTs (2004-2014).
|
Discussion
This long term follow-up of a cohort of children
vaccinated with a single dose of live attenuated HAV vaccine in 2004
demonstrated a seroprotection rate of 98.1% (anti – HAV GMT of
100.46mIU/mL.), 10 years after vaccination, in those who showed adequate
seroprotection in earlier studies. The seroprotection rate would be
87.6%, on inclusion of 13 participants who had to be given additional
hepatitis A doses because of poor immunogenicity documented in earlier
evaluations [6,7]. None reported hepatitis-like illness.
The strength of this study is that 85% of the cohort
enrolled in 2004 reported regularly for follow-up. Differences in the
kits used for evaluation of titers is one of the limitation of this
study. In the first three follow-ups, immunogenicity was assessed using
quantitative Axsym HAVB 2.0 ELISA from Abbott Laboratories (Abbott Park,
Illinois, USA). These kits were not available in 2014, and hence
different kits had to be used for the present analysis. AxSYM
HAVB
2.0 uses microparticle enzyme immunoassay (MEIA) whereas present kits
were based on ECLIA technology [8]. Some reports have shown higher
sensitivity of ECLIA as compared to ELISA-based immunoassays [9], and
this could be a reason for the higher titers of antibodies observed in
this analysis. Cross validation of immunogenicity data between the two
kits was not possible as sera from previous analyses were not available.
Another limitation was the cohort contamination with second dose of the
vaccine. Due to ethical reasons, children who remained seronegative
after vaccination in 2004 were given 2 doses of inactivated (licensed)
HAV vaccine; in 2007 those who remained seronegative received a second
dose of now licensed live HAV vaccine, In 2010, however, in view of the
encouraging reports of persisting immunological memory despite low
titres [10,11], no revaccination was done in seronegative children. The
contribution of asymptomatic infections towards immunogenicity also
cannot be ruled out as there was no control group in our study.
Our results are consistent with earlier reports from
China [4,5], which showed seroprotection levels of 98.6%, 93.6%, 83.3%
and 80.2% at 2 months, 12 months, 6 years and 10 years, respectively.
Although studies have reported higher seroprotection and GMT levels with
two dose schedule of live HAV vaccine, long-term efficacy studies with a
single dose have uniformly demonstrated complete protection against the
disease suggesting anamnestic response despite low titers [10].
Similarly, anamnestic response (immunological memory) is also the basis
for recently proposed single dose schedule even for inactivated HAV
vaccine [1,11,12]. The long-term results of the other Indian study (multicentric)
of a single dose live HAV vaccine [13], are awaited.
In conclusion, a single dose of live attenuated HAV
vaccine continues to show excellent immunogenicity 10 years after
immunization, in Indian children. A single dose of live HAV vaccine will
cut costs considerably, while providing long-term protection.
Acknowledgements: Dr Anand Pandit and Dr. Ganesh
Kadhe for their help and guidance; Dr Shivali Arora for technical
assistance; Pediatric Research team at KEM Hospital Research Centre for
ensuring follow-up.
Contributors: SB, AS, AB: designed the
study, recruited patients, analyzed results and wrote the manuscript;
VK, AM: provided technical help needed for the study. All authors
approved the final version of manuscript.
Funding: Wockhardt Ltd; Competing Interests:
AM and VK are paid employees of Wockhardt Ltd. SAB, AS and AB received
investigator fee for conduct of the study.
|
What This Study Adds?
• A single dose of live attenuated HAV
vaccine is immunogenic in 87.6% of children at 10 years after
vaccination.
|
References
1. WHO position paper on hepatitis A vaccines: June
2012-Recommendations. Vaccine. 2013;31:285-6.
2. Patwari AK, Shah N. Hepatitis A vaccines. In:
Vashishtha VM, Choudhury P, Bansal CP, Yewale VN, Agarwal R, editors.
IAP Guidebook on Immunization 2013-14. 1st ed. Gwalior: National
Publication House; 2014. p. 249-56.
3. Vashishtha VM, Choudhury P, Kalra A, Bose A,
Thacker N, Yewale VN, et al. Indian Academy of Pediatrics (IAP)
Recommended Immunization Schedule for Children Aged 0 through 18 years –
India, 2014 and Updates on Immunization. Indian Pediatr.
2014;51:785-800.
4. Mao JS, Chai SA, Xie RY, Chen NL, Jiang Q, Zhu XZ,
et al. Further evaluation of the safety and protective efficacy of
live attenuated hepatitis A vaccine (H2-strain) in humans. Vaccine.
1997;15:944-7.
5. Zhuang FC, Qian W, Mao ZA, Gong YP, Jiang Q, Jiang
LM, et al. Persistent efficacy of live attenuated hepatitis A
vaccine (H2-strain) after a mass vaccination program. Chin Med J (Engl).
2005;118:1851-6.
6. Bhave S, Bavdekar A, Madan Z, Jha R, Bhure S,
Chaudhari J, et al. Evaluation of immunogenicity and tolerability
of a live attenuated hepatitis A vaccine in Indian children. Indian
Pediatr. 2006;43:983-7.
7. Bhave S, Bavdekar A, Sapru A, Bawangade S, Pandit
A. Immunogenicity of single dose live attenuated hepatitis A vaccine.
Indian Pediatr. 2011;48:135-7.
8. Cobas e 411 Analyzer. Available from:
www.roche-diagnostics.hu/fmfiles/re7193001/Hungary/roche.hu /Diagnostic/Termekek/CobasE411/cobas_e_411_
EN.pdf. Accessed October 7, 2014.
9. Zhang QY, Chen H, Lin Z, Lin JM. Comparison of
chemiluminescence enzyme immunoassay based on magnetic microparticles
with traditional colorimetric ELISA for the detection of serum
á-fetoprotein. J Pharm Analysis. 2012;2:130-5.
10. Wang XY, Xu ZY, Ma JC, von Seidlein L, Zhang Y,
Hao ZY, et al. Long-term immunogenicity after single and booster
dose of a live attenuated hepatitis A vaccine: results from 8-year
follow-up. Vaccine. 2007;25:446-9.
11. Fiore AE, Feinstone SM, Bell BP. Hepatitis A
vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors.
Vaccines. 5th ed. Philadelphia, PA: Elsevier Inc.; 2008. p. 191.
12. Ott JJ, Wiersma ST. Single-dose administration of
inactivated hepatitis A vaccination in the context of hepatitis A
vaccine recommendations. Int J Infect Dis. 2013;17:e939-44.
13. Faridi MM, Shah N, Ghosh TK, Sankaranarayanan VS,
Arankalle V, Aggarwal A, et al. Immunogenicity and safety of live
attenuated hepatitis A vaccine: A multicentric study. Indian Pediatr.
2009;46:29-34.
|
|
|
 |
|

