|
|
|
Indian Pediatr 2012;49:
621-625 |
 |
Validity of Two Point-of-Care Glucometers in
the Diagnosis of Neonatal Hypoglycemia
|
|
S Ngerncham, S Piriyanimit, T Kolatat, P Wongsiridej, L Inchgarm, R
Kitsommart,
P Vutrapongwatana and K Jeerapaet
From Division of Neonatology, Department of
Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University.
Bangkok, Thailand.
Correspondence to: Dr Sopapan Ngerncham, Assistant
Professor, Division of Neonatology, Department of Pediatrics,
Faculty of Medicine Siriraj Hospital, Mahidol University, 1 Prannok
Road, Bangkoknoi, Bangkok,
Thailand.
Email: [email protected] ,
[email protected]
Received: March 26, 2011;
Initial review: May 23, 2011;
Accepted: October 14, 2011.
Published online: January 17, 2012.
SII: S097475591100260-1
|
Objective : To
estimate validity of two point-of-care glucometers for the diagnosis of
neonatal hypoglycemia and to determine the glucometer’s cut-off values
for which standard laboratory confirmatory test are no longer needed.
Design: Prospective study.
Settings: A tertiary care, university hospital in
Bangkok, Thailand.
Participants: The study included 180 blood
specimens from 166 high-risk neonates aged between 1-24 hours.
Results: On average, most of the blood glucose
read-outs from the Nova StatStrip and SureStep were higher than
laboratory plasma glucose throughout the glucose range with mean
differences (SD) of 11.2 (8.4) mg/dL and 13.7 (6.8) mg/dL, respectively.
Sensitivity of Nova StatStrip and SureStep were 62% and 53.3%,
respectively. Specificity and positive predictive value of both
glucometers were 100%. Negative predictive values of both glucometers
were approximately 85%. The cut-off levels with 100% negative predictive
values were 63 mg/dL and 62 mg/dL for Nova StatStrip and SureStep,
respectively.
Conclusions: None of the glucometers in this
study has sufficient validity to replace laboratory testing in
diagnosing hypoglycemia. Confirmatory plasma glucose for diagnosis of
hypoglycemia is needed when POC readings are between 39 and 63 mg/dL for
Nova StatStrip and between 39 and 62 mg/dL for SureStep.
Key words: Glucometer, Neonatal hypoglycemia, Point-of-care
test.
|
|
Hypoglycemia in neonates is an
emergency condition requiring immediate treatment to prevent serious
outcomes [1]. Most of hypoglycemic infants are asymptomatic; hence,
screening is needed for high-risk infants. There are many portable
glucometers available for point-of-care (POC) testing. However, accuracy
testing of these machines is usually done in older children and adults
with diabetes mellitus. Operational glucose levels in newborn period run
in a lower range than those in diabetic patients [2]. The number of
accuracy studies in newborn infants is increasing, with promising
results [3-7]. At Siriraj Hospital, POC glucose testing is done using
the OneTouch SureStep
Hospital Test Strips. Presently, there is no data available on their
validity in neonates. The Nova StatStrip
has recently been launched, and reported to have
demonstrable accuracy with interference corrections [8]. We performed
this study to estimate validity of two POC glucometers for the diagnosis
of neonatal hypoglycemia and to determine the glucometers’ cut-off
values for which standard laboratory confirmatory tests are no longer
needed.
Methods
This was a prospective study approved by the Ethics
Committee of the Faculty of Medicine, Siriraj Hospital and informed
consents were obtained prior to the study. The study was conducted at
Siriraj Hospital, which is a tertiary care university hospital. We
consecutively recruited high-risk newborn infants aged between 1 and 24
hours from the High-risk nursery and the Intermediate care unit. The
patients included small for gestational age, large for gestational age,
infant of diabetic mother, and low birth weight infants. Critically ill
infants in the NICU were excluded due to possible multiple medication
administration. The study was conducted only during office hours, in
order to limit the POC glucose testing to that of trained operators,
which were two pediatric residents and a technician.
This was a split-sample design using single venous
blood sample from a peripheral vein for both the glucometers and the
reference laboratory. Two glucometers were used in the study. The first
one was the OneTouch SureStep
Hospital Test Strips (LifeScan, Inc., a Johnson & Johnson
Company, Milpitas, CA, USA), with a photometric glucose oxidase system.
The other was the Nova StatStrip (Nova Biomedical, Waltham,
Massachusetts, USA), with a modified glucose oxidase-based amperometric
system. The POC glucose testing was done by trained operators
immediately after the blood was drawn. Cuvette tube with sodium fluoride
as a stabilizer was used for reference laboratory plasma glucose
testing. A Roche Modular P 800 (Roche Diagnostics (Thailand) Ltd.) with
enzymatic colorimetric assay using hexokinase enzyme was used for
measuring reference plasma glucose. The reference laboratory had
International Standard Organization (ISO) 15189 certification. The
quality control materials were run according to manufacturer’s
recommendations. The laboratory plasma glucose tests had a bias of 1.75%
and imprecision (%CV) of 1.62%. The blood specimens were tested for
plasma glucose within one hour after being drawn. The readers of the
index tests (POC glucometers) and the reference standard were blinded to
the results of each other at the time they read the results from the
device. Hypoglycemia was defined as laboratory plasma glucose less than
40 mg/dL [9].
Demographic data, hematocrit level on the same day of
blood glucose testing, and time difference between blood sampling and
blood test were recorded. Sample size calculation was based on
sensitivity and specificity of at least 93% with acceptable error of 7%.
According to the incidence of hypoglycemia in high-risk infants at our
hospital (30%), we required at least 174 tests.
Statistical analysis: The SPSS 17.0 (SPSS Inc,
Chicago, IL, USA) was used for statistical analysis. Differences of
glucose level between POC glucose testing and laboratory testing were
presented as mean difference. Following the standard DIN EN ISO 15197,
the differences were presented as percentage within ± 15 mg/dL and
percentage within ± 20% of the reference method for plasma glucose <75
mg/dL and ³75
mg/dL, respectively [10]. The differences were also presented as
percentage within ±15 mg/dL of the reference method for plasma glucose
<99 mg/dL per National Committee for Clinical Laboratory
Standards (NCCLS) recommen-dation [11] and percentage within ±10%
for plasma glucose ranging from 30 to 400 mg/dL [12].
Sensitivity, specificity, positive and negative
predictive values (PPV and NPV) for detection of hypoglycemia were
determined. ROC curves of both glucometers were plotted. The pre-test
probability (prevalence) of hypoglycemia in high-risk neonates at our
hospital was 30%. We calculated PPV and NPV from sensitivity and
specificity because the incidence of hypoglycemia in this study was 51%,
which was higher than our population.
Results
The study period was between December 1, 2008 and May
31, 2009. There were 166 infants (61% males) recruited with 180 sets of
blood specimens. Mean (SD) gestational age and birthweight were 37.1
(2.8) weeks and 2,799.6 (837.3) grams, respectively. Mean (SD)
hematocrit on the day of the study was 52.0% (7.2%). Range (minimum,
maximum) and median age (P25, P75) at blood drawn were 23 (1, 24) and
1.0 (1.0, 1.0) hour, respectively. Approximately 31% and 91% of the
blood specimens had glucose measurement performed at the laboratory
within 30 minutes and 60 minutes of blood drawn, respectively. Mean (SD)
time difference between blood drawn and laboratory plasma glucose
measurement was 44.5 (24.7) minute.
Of 180 samples, 92 (51%) were diagnosed with
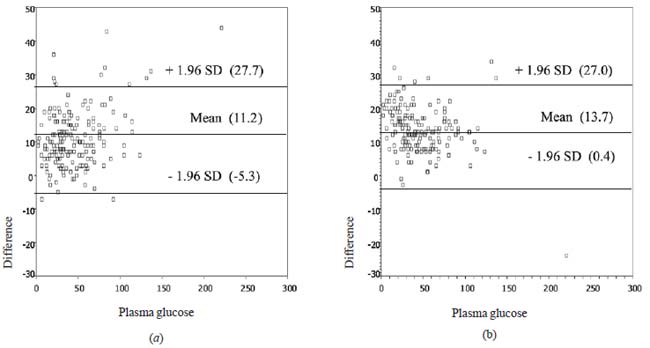
hypoglycemia. Blood glucose levels measured by different methods are
shown in Web Table I. Differences between
blood glucose from each POC glucometer and from laboratory plasma
glucose were normally distributed, thus 95% of the measurements had
differences between mean plus and minus 1.96 SD. On average, most of the
blood glucose read out from the Nova StatStrip and SureStep were higher
than laboratory plasma glucose throughout the glucose range with mean
differences (SD) of 11.2 (8.4) mg/dL and 13.7 (6.8) mg/dL, respectively
(Fig. 1). Results presented following the standard DIN EN
ISO 15197, NCCLS and ADA are in Web Table II.
 |
|
Fig. 1 Scatter plots of differences
between laboratory plasma glucose and (a) Nova StatStrip and (b)
SureStep.
|
When using POC glucometer to diagnose hypoglycemia,
the sensitivity of Nova StatStrip was higher than SureStep but both were
less than 70%. The specificities and PPV were 100% for both POC
glucometer testings. Negative predictive value of both POC glucometers
were less than 90% (Table I).
TABLE I Sensitivity, Specificity, Positive and Negative Predictive Values for Detecting Different Plasma Glucose Levels*
|
Cut-off level of plasma glucose (mg/dL) |
Nova StatStrip
|
SureStep
|
|
Sn |
Sp |
PPV† |
NPV† |
Sn |
Sp |
PPV† |
NPV† |
|
< 40 |
62.0 |
100 |
100 |
85.7 |
53.3 |
100 |
100 |
83.4 |
|
< 52 |
92.4 |
90.9 |
82 |
96.6 |
93.5 |
95.5 |
89.3 |
96.8 |
|
Nova StatStrip<63 |
100 |
65.9 |
55.7 |
100 |
– |
– |
– |
– |
|
SureStep< 62 |
– |
– |
– |
– |
100 |
70.5 |
59.3 |
100 |
|
* Data are presented as percentage; †
Positive predictive value (PPV) and negative predictive value
(NPV) were calculated from sensitivity (Sn), specificity (Sp)
and 30% incidence of hypoglycemia; Hypoglycemia: plasma glucose
<40 mg/dL. |
From ROC curve (not shown), cut-off levels that would
yield the best sensitivity and specificity for both POC glucometers were
less than 52 mg/dL, with NPV of approximately 97% (Table I).
To diagnose blood glucose of less than 45 mg/dL, the sensitivity of both
glucometers were 72% and 61%, respectively (Table II).
From ROC curve (not shown), cut-off levels that would yield the best
sensitivity and specificity for Nova StatStrip and SureStep to diagnose
blood glucose of less than 45 mg/dL were less than 60 mg/dL and less
than 55 mg/dL, respectively (Table II).
TABLE II Sensitivity, Specificity, Positive and Negative Predictive Values for Detecting Different Plasma
Glucose Levels* (For Therapeutic Goal of Plasma Glucose At 45 mg/dL).
|
Cut-off level of plasma glucose (mg/dL) |
Nova StatStrip
|
SureStep
|
|
Sn |
Sp |
PPV† |
NPV† |
Sn |
Sp |
PPV† |
NPV† |
|
<45 |
71.7 |
98.6 |
95.8 |
89.1 |
61.3 |
100 |
100 |
85.8 |
|
< 60 |
98.1 |
86.5 |
75.7 |
99.1 |
– |
– |
– |
– |
|
< 55 |
– |
– |
– |
– |
92.5 |
98.6 |
96.7 |
96.8 |
|
Nova StatStrip <63 |
100 |
78.4 |
66.5 |
100 |
– |
– |
– |
– |
|
SureStep <69 |
– |
– |
– |
– |
100 |
71.6 |
60.2 |
100 |
|
* Data are presented as percentage; † Positive predictive
value (PPV) and negative predictive value (NPV) were calculated
from sensitivity (Sn), specificity (Sp) and 30% incidence of
hypoglycemia. |
Discussion
Our results agree with previous studies which
concluded that glucose reagent strips should be considered only as a
screening test, not as a diagnostic test, due to their questionable
reliability [2,6,7]. Though, the difference of glucose level
between both POC glucometers and laboratory is normally distributed, 95%
of the differences lie in a wide range (between -5.3 and 27.7 mg/dL for
Nova StatStrip and between 0.4 and 27.0 mg/dL for SureStep), and are not
acceptable for clinical purposes.
Validity of POC glucometer also depends on
pre-analytical processes. Using sample tubes containing sodium fluoride
(NaF) has been recommended in order to minimize ex-vivo glycolysis [13,
14]. Such a method was adopted in our study. Though fluoride is the best
available preservative for blood glucose measurement, the antiglycolytic
action may delay for up to 4 hours [15]. The mean plasma glucose
concentration could decrease by 4.3% at 1 hour and by 4.6% at 2 hours in
blood kept in tube containing NaF [15,16]. Our results are not
applicable for different glucose preservative used or different glucose
measurement process.
Mann, et al. [17] found that error rates for
low hematocrit between 25% and 34% ranged from 16.4% to 18.4%.
Hematocrit of the infants recruited in our study ranges from 27% to 65%
which is within the operational range of accuracy (25-65%) for both POC
glucometers. There were only three specimens with hematocrit less than
34% in our study. Hence, glucometer error secondary to low hematocrit
should not be a major problem in this study.
Based on the standard DIN EN ISO 15197: at blood
glucose concentrations <75 mg/dL, ³95%
of the blood glucose results should fall within ±15 mg/dL of the
reference method and at blood glucose concentrations
³75 mg/dL,
³95% of the blood
glucose results should fall within ±20% [10]. Nova StatStrip was more
accurate than SureStep at glucose level <75 mg/dL, although neither of
them met the minimum requirement of DIN EN ISO 15197.
NCCLS recommended that discrepancies in blood glucose
measurements should be less than 15 mg/dL when actual glucose
concentrations were less than 99 mg/dL. Subsequently, 75.3% of Nova
StatStrip fulfilled such standard, compared to 61.8% of SureStep [11].
More-over, using the ADA standard, none of the POC glucometers tested
demonstrated satisfactory accuracy. Nova StatStrip and SureStep had only
22.6% and 9.6% of their tests achieved a total error of less than 10% at
glucose concentration ranging from 30 to 400 mg/dL [12].
In clinical practice, sending for confirmation plasma
glucose in all cases would add to the expense, considering how often
glucose levels are assessed in newborns. Using POC glucometer alone, we
may miss 38% and 47% of hypoglycemia (POC glucose level <40 mg/dL) by
Nova StatStrip and SureStep, respectively. With ROC analysis, the
cut-off level with the best sensitivity and specificity of both POC
glucometers are 52 mg/dL. At this cut-off level, we will still miss
approximately 6-8% of hypoglycemia. For POC glucose reading of less than
40 mg/dL, we could be confident of the diagnosis of hypoglycemia without
sending confirmatory plasma glucose. Thus, confirmatory plasma glucose
for diagnosis of hypoglycemia would be required when POC glucose
readings are between 39 and 63 mg/dL for Nova StatStrip and between 39
and 62 mg/dL for SureStep.
Practically, different blood glucose levels are used
as the "operational threshold" and the "therapeutic objective" [2].
Mostly accepted therapeutic goal is to keep blood glucose higher than 45
mg/dL [2,13]. For this purpose of defining blood glucose less than 45
mg/dL, the cut-off levels that confirmatory blood glucose is not
necessary would be different. Confirmatory plasma glucose for diagnosis
of blood glucose less than 45 mg/dL is needed when POC glucose readings
are between 44 and 63 mg/dL for Nova StatStrip and between 44 and 69 mg/dL
for SureStep.
The clinical application of our study is limited to
clinically stable infants without medications that might interfere with
glucose measurement. The participants in this study were infants aged
between 1 and 24 hours. If the POC glucose measurement is done in older
infants, the clinicians must be aware of the possibility of
hyperbilirubinemia interfering with glucose measurement.
Both POC glucometers in this study did not meet the
minimum requirement of DIN EN ISO 15197, NCCLS, and ADA. A degree of
caution should be exercised in the interpretation of POC glucose
measurements as they may not possess sufficient accuracy to replace
laboratory plasma glucose results. Individual devices may need their own
operational cut-off values for sending plasma glucose for confirmation.
Acknowledgements: Dr Chulaluk Komoltri for
helping in statistical analysis.
Contributors: NS, PS, KT, WP, KR and VP:
Conception and design, analysis and interpretation of data, drafting the
manuscript, critical revision of the manuscript for important
intellectual content and final approval of the version to be published;
IL: Acquisition of data, critical revision of the manuscript for
important intellectual content, final approval of the version to be
published.
Funding: This study was supported by Siriraj
Routine to Research Management Fund. The strips of Nova StatSripTM were
provided by Meditop Co, Ltd, Bangkok, Thailand;
Competing interests: None stated.
|
What is Already Known?
• Point-of-care glucometer should be
considered only as a screening test, not as a diagnostic test in
diagnosing neonatal hypoglycemia.
What This Paper Adds?
• Each individual glucometer needs its own validity study and
operational cut-off values for the decision to send laboratory
plasma glucose for confirmation.
|
References
1. Burns CM, Rutherford MA, Boardman JP, Cowan FM.
Patterns of cerebral injury and neurodevelopmental outcomes after
symptomatic neonatal hypoglycemia. Pediatrics. 2008;122:65-74.
2. Cornblath M, Hawdon JM, Williams AF, Aynsley-Green
A, Ward-Platt MP, Schwartz R, et al. Controversies regarding
definition of neonatal hypoglycemia: suggested operational thresholds.
Pediatrics. 2000;105:1141-5.
3. Innanen VT, DeLand ME, deCampos FM, Dunn MS.
Point-of-care glucose testing in the neonatal intensive care unit is
facilitated by the use of the Ames Glucometer Elite electrochemical
glucose meter. J Pediatr. 1997;130:151-5.
4. Leonard M, Chessall M, Manning D. The use of a
Hemocue blood glucose analyser in a neonatal unit. Ann Clin Biochem.
1997;34:287-90.
5. Girouard J, Forest JC, Masse J, Leroux M, Bradburn
NC, Noblet TC, et al. Multicenter evaluation of the Glucometer
Elite XL meter, an instrument specifically designed for use with
neonates. Diabetes Care. 2000;23:1149-53.
6. Ho HT, Yeung WK, Young BW. Evaluation of "point of
care" devices in the measurement of low blood glucose in neonatal
practice. Arch Dis Child Fetal Neonatal Ed. 2004;89:F356-9.
7. Michel A, Kuster H, Krebs A, Kadow I, Paul W,
Nauck M, et al. Evaluation of the Glucometer Elite XL device for
screening for neonatal hypoglycaemia. Eur J Pediatr. 2005;164:660-4.
8. Holtzinger C, Szelag E, Dubois JA, Shirey TL,
Presti S. Evaluation of a new POCT bedside glucose meter and strip with
hematocrit and interference corrections. Point of Care. 2008;7:1-6.
9. Scher MS. Seizures in neonates. In: Martin
RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s neonatal-perinatal
medicine: diseases of the fetus and infant. Philadelphia: Mosby
Elsevier; 2006. p. 956-76.
10. Mahoney JJ, Ellison JM. Assessing glucose monitor
performance—a standardized approach. Diabetes Technol Ther.
2007;9:545-52.
11. Sacks DB, Bernhardt P, Dunka LJ, Goldstein DE,
Hortin GL, Mueller P. Point-of-Care Blood Glucose Testing in Acute and
Chronic Care Facilities; Approved Guideline. 2002. Available from: URL:
http://www.clsi.org/source/orders/free/c30-a2.pdf. Accessed on March 24,
2011.
12. American Diabetes Association. Self-monitoring of
blood glucose. Diabetes Care. 1994;17:81-6.
13. Adamkin DH. Postnatal glucose homeostasis in
late-preterm and term infants. Pediatrics. 2011;127:575-9.
14. World Health Organization. Use of anticoagulants
in diagnostic laboratory investigations 2002; Available from: URL:
http://whqlibdoc.who.int/hq/2002/WHO _DIL_LAB_99.1_Rev.2.pdf. Accessed
on March 24, 2011.
15. Chan AY, Swaminathan R, Cockram CS. Effectiveness
of sodium fluoride as a preservative of glucose in blood. Clin Chem.
1989;35:315-7.
16. Gambino R, Piscitelli J, Ackattupathil TA,
Theriault JL, Andrin RD, Sanfilippo ML, et al. Acidification of
blood is superior to sodium fluoride alone as an inhibitor of glycolysis.
Clin Chem. 2009;55:1019-21.
17. Mann EA, Salinas J, Pidcoke HF, Wolf SE, Holcomb
JB, Wade CE. Error rates resulting from anemia can be corrected in
multiple commonly used point-of-care glucometers. J Trauma.
2008;64:15-20.
|
|
|
 |
|

