|
|
|
Indian Pediatr 2011;48:
613-617 |
 |
Effect of High-dose Phenobarbital on Oxidative
Stress in Perinatal Asphyxia: An Open Label Randomized
Controlled Trial |
|
Geeta Gathwala, Ashish Marwah, Veena Gahlaut* and Poonam Marwah
From the Neonatal Services Division, Department of
Pediatrics; and *Department of Biochemistry;
Pt BD Sharma PGIMS,
Rohtak, India.
Corresponding Author: Dr Geeta Gathwala, Medical Enclave,
Pt BD Sharma PGIMS, Rohtak 124 001,Harayana, India.
Email:
g [email protected]
Received: November 12, 2009;
Initial review: January 11, 2010;
Accepted: July 27, 2010.
Published online: 2010 November 30.
PII: S097475590900675-1
|
Objective: To evaluate the effect of high dose
phenobarbital on lipid peroxidation and antioxidant enzymes in perinatal
asphyxia.
Design: Open label, Randomized controlled
trial.
Setting: Neonatal intensive care unit of a tertiary
care teaching hospital.
Participants: 72 full term inborn neonates
with severe birth asphyxia.
Methods: Neonates were randomized to Study (phenobarbital)
group and Control group. The infants in the study group received
phenobarbital infusion (40mg/kg) within first two hours of life while
babies in the control group did not receive any phenobarbital. Rest of the
management in both the groups was as per the unit protocol for the
management of hypoxic ischemic encephalopathy. A cerebrospinal fluid
examination was done at 12 ± 2 hours of life to determine the levels of
superoxide dismutase, glutathione peroxidise and malonyldialdehyde. 60
neonates were followed up at 1 month of age when a detailed neurological
examination was done.
Results: Four neonates in the study group and six
neonates in the control group died during the study. Two neonates in the
study group were lost to follow up. The cerebrospinal fluid lipid
peroxides and antioxidant enzymes were significantly lower in the
phenobarbital group as compared to the control group. The neurological
outcome at one month follow up was found to be comparable between the two
groups.
Conclusion: Phenobarbital (40mg/kg) given in the
first two hours of life in term neonates with perinatal asphyxia led to a
decrease in CSF levels of lipid peroxides and antioxidant enzymes at 12 ±
2 hours of life.
Key words: Antioxidants, Management, Perinatal asphyxia,
Phenobarbital.
|
|
N
euronal injury and the subsequent
neuronal death during hypoxic ischemic encephalopathy (HIE) occurs by two
basic mechanisms viz, rapid cell death and delayed cell death. The
former occurs within minutes, is caused by glutamate receptor activation
leading to increased sodium entry followed by a passive influx of chloride
ions down its electro-chemical gradient along with water, causing cell
swelling and lysis. The delayed cell death occurs over hours to even days
and is caused by activation of N-methyl-d-aspartate (NMDA) receptors
leading to entry of calcium intracellularly and the subsequent activation
of several degrading enzymes such as phospholipases, nucleases, proteases
etc, causing cell injury and death [1-5]. Institution of therapies post
asphyxia (during the critical first six hours) have been found to be
neuroprotective.
Phenobarbital with its established safety profile and
low cost may hold promise as a neuroprotective agent. Its major mechanism
of action is its free radical scavenging action, suppression of cerebral
oxidative metabolism and the blunting of cerebral excitotoxicity by
depressing glutamate responses within the brain [6-8]. The present study
was planned to evaluate the effect of phenobarbital on lipid peroxidation
and antioxidant enzymes in term neonates with perinatal asphyxia.
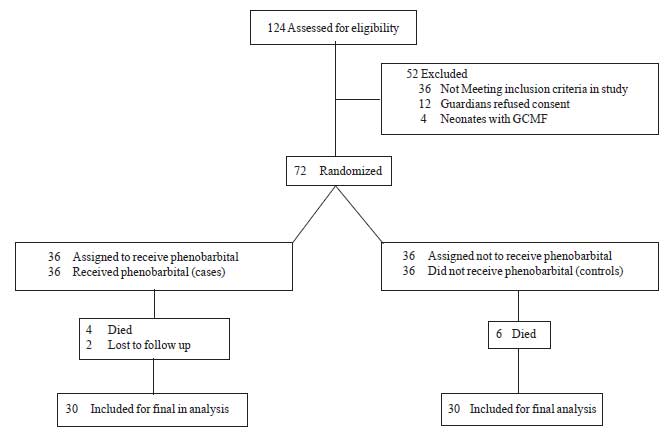 |
|
Fig.1 Study flow diagram.
|
Methods
The study was conducted from 1 May, 2006 to 30th
October, 2007 in the Neonatal Services Division, Department of Pediatrics
and Biochemistry of a tertiary care teaching institution. Full term inborn
babies with severe birth asphyxia who met the selection criteria
(umbilical vein cord blood pH<7 and APGAR score <6 at 5 minutes) were
randomized to the Study (n=36) and the Control group (n=36)
using a random number table. Random number sequences were placed in opaque
sealed envelopes which were opened once the baby had been resuscitated and
met the selection criteria. The babies in the study group received
Phenobarbital (40mg/kg) as an intravenous infusion over 60 minutes within
the first 2 hours of life under continuous monitoring for heart rate,
oxygen saturation, respiration and mean arterial pressure. There was no
blinding and the control group received no placebo. Rest of the management
in both the groups was as per the unit protocol for the management of HIE.
An informed consent was obtained from the parents of all the neonates and
the study was cleared by the hospital ethics committee.
Under all aseptic precautions, a cerebrospinal fluid (CSF)
examination was done at 12 ± 2 hours of life in all the babies to
determine the levels of lipd peroxides (malonyldialdehyde, MDA) and
anti-oxidant enzymes, (superoxide dismutase and glutathione peroxidise
[SOD, GPx]) [9-11] and CSF cell count. Protein and glucose estimation was
also done to rule out meningitis. The staging of HIE was done according to
the criteria of Sarnat and Sarnat [12]. Cranial ultrasound was done for
all babies on day 3 and day 7 of life. Details of neonatal seizures were
recorded and a detailed neurological examination was done at the time of
discharge. Follow-up was done at 1 month of age when a detailed
neurological examination was done and a MRI brain and an EEG were
obtained.
The statistical tests used for the analysis were the
unpaired student’s ‘t’ test and the chi-square test.
Results
The baseline data including gestational age, birth
weight, APGAR score and cord pH were comparable between the two groups (Table
I). The phenobarbital infusion was well tolerated and the
temperature, heart rate, mean arterial pressure (MAP) and oxygen
saturation during the infusion were within the normal limits. Six babies
in the phenobarbital group and 11 in the control group received oxygen
(target SPO 2 90-95%).Three babies in
the phenobarbital group and four in the control group were ventilated.
These data were comparable between the two groups. Four babies in the
phenobarbital group and six babies in the control group died during the
study. Two babies in the phenobarbital group were lost to follow up.
TABLE I
Comparison of Baseline Data Between The Study and The Control Group
|
Parameters |
Study Group |
Control Group |
| |
(n=30) |
(n=30) |
| |
(Mean ± SD) |
(Mean ± SD) |
|
Birthweight (kg) |
3.00 ± 0.17 |
2.91 ± 0.15 |
|
Gestational age (wks) |
38.35 ± 1.40 |
39.35 ± 1.22 |
| APGAR
score at 1 min |
2.1 ± 0.75 |
1.93 ± 0.78 |
| At
five minutes |
4.7 ± 0.53 |
4.27 ± 0.74 |
| pH (at
birth)* |
6.90 ± 1.06 |
6.88 ± 0.09 |
| HIE
I |
7 |
4 |
|
II |
17 |
15 |
|
III |
6 |
11 |
| Neonatal
seizures†# |
52 (24-120) h |
78 (24-160) h |
|
* umbilical vein cord blood pH; #Median time to
become passive (range); † P <0.05; HIE: hypoxic ischemic
encephalopathy. |
The mean CSF MDA level and the mean CSF SOD and GPx at
12 ± 2 hours of life was significantly lower at in the Phenobarbital group
as compared to the Control group (P<0.001) (Table II).
Seizures were controlled and became passive at day three (median 52
hours, range 24-120 hours)in the Phenobarbital group compared to day four
(median 78 hours, range 24-160 hours) in the Control group (P<0.05).
The neurological outcome at one month assessed on neurological
examination, MRI brain and EEG was similar in the two groups.
TABLE II
Oxygen Free Radical and Antioxidant Enzymes Levels in CSF at 12 ± 2 Hours of Life
|
Parameters |
Study Group |
Control Group |
| |
(n=30) |
(n=30) |
|
MDA (nmol/mg protein) |
1.07 ± 0.14 |
1.33 ± 0.10 |
|
SOD (Eu/mg protein) |
4.34 ± 0.93 |
6.98 ± 1.19 |
|
GPx (µmoles of NADPH oxidized/min/mg protein) |
5.36 ± 0.92 |
7.31 ± 1.59 |
|
P value <0.001; all values in mean ± SD; MOD:
Malonyldialdehyde; SOD: Superoxide dismutase; GPx: Glutathione
Peroxidase. |
Discussion
The mean CSF lipid peroxide (MDA) and antioxidant
levels (SOD, GPx) were found to be significantly lower in the
phenobarbital group as compared to the control group. Phenobarbital
infusion at 40 mg/Kg was well tolerated by all neonates. Singh, et al.
[13], in a recent study, administered phenobarbital in a dose of 20 mg/kg
within first six hours of life to near term neonates (>34 weeks) post
asphyxia and reported similar findings [13].
MDA is produced as a result of lipid peroxidation and
lower values of MDA imply a reduction in free radical production, possibly
by phenobarbital. The significantly higher levels of antioxidant
enzymes (SOD, GPx) in the Control group as compared with Phenobarbital
group was possibly due to a compensatory increase in response to the
higher levels of lipid peroxidation and free radical damage in the control
group.
The incidence of neonatal seizures in the Phenobarbital
group was comparable to that in the control group. However, the mean
duration of seizures was significantly lower in the Phenobarbital group as
compared to the Control Group. The neurological outcome at one month of
age of neonates in the Phenobarbital group was; however, not different
from the Control group.
Singh, et al. [14] in their study on 45 term and
near term infants (>34 weeks gestation) post asphyxia administered
phenobarbital (20mg/kg) within 6 hours of life to 25 neonates (20
controls). The study showed a significant decrease in the incidence of
seizures in the phenobarbital group (8%) as compared to the controls
(40%). However, it did not alter the mortality or neurological outcome at
discharge [14].
Hall, et al. [15] in their study on 40 term
newborn infants with severe birth asphyxia administered phenobarbital
(40mg/kg) within first six hours post asphyxia and showed a 27% reduction
in the incidence of seizures in the phenobarbital group as compared to
control group, although the difference was statistically not significant.
The incidence of seizures in the present study was similar, but the time
taken for seizures to become passive was significantly lesser in the study
group. In their study, the neurological outcome at 3 years of age was
normal in 73.3% in the Phenobarbital group (n=15) compared to only
18.7% in the control group(15). We found comparable neurological outcome
in the two groups. However, as the follow up period in this study was only
one month, a longer follow up possibly would have better elicited the
differences on neurological examination. Svenningsen, et al. [16]
had previously reported a significantly better neurological outcome at 1˝
years of age in their study on full term babies with severe birth asphyxia
who received phenobarbital (20mg/kg) within first 24 hours of life.
Recently, Evans, et al. [17] reviewed the
efficacy of phenobarbital in term infants following perinatal asphyxia on
death or subsequent severe neurodeve-lopmental disability and or the
prevention of seizures. They analyzed all randomised or quasi randomised
controlled trials which reported data comparing mortality,
neurodevelopmental disability, neonatal seizures and adverse events,
following phenobarbital in term infants compared to controls (with or
without placebo) following perinatal asphyxia and concluded that
barbiturates when compared to conventional therapy following peri-natal
asphyxia demonstrated no difference in risks of death, severe
neurodevelopmental disability, or the combined outcome of death or severe
neuro-developmental disability [17].
The results from the present study showed that
phenobarbital (40mg/kg) given to full term babies with severe birth
asphyxia within the first 2 hours of life was safe and well tolerated. It
led to a statistically significant reduction in CSF lipid peroxidation (MDA)
and subsequent free radical injury and antioxidant enzyme (SOD, GPx)
levels but was not associated with any significant improvement in the
neurological outcome assessed at one month follow up. This could form a
strong basis for conducting a larger study with a longer follow up to
better document the neuroprotective role of phenobarbital in perinatal
asphyxia.
Contributors: GG conceived and designed the study
and revised the manuscript for important intellectual content. She will
act as guarantor of the study. AM collected data and drafted the paper. VG
and AM conducted the laboratory tests and interpreted them. PM researched
the literature and contributed to manuscript writing. The final manuscript
was approved by all authors.
Funding: None
Competing interests: None stated.
|
What is Already Known?
• Lipid peroxidation and oxygen free radical
injury is involved in neuronal injury in perinatal asphyxia and
HIE.
What This Study Adds?
• Phenobarbital in a dose of 40mg/kg
administered in the first two hours of life decreased CSF lipid
peroxide and antioxidant enzyme levels at 12±2 hours of life in
neonates with perinatal asphyxia.
|
References
1. Volpe JJ. Hypoxic ischemic encephalopathy:
biochemical and physiological aspect. In: Neurology of the Newborn,
3rd ed. Philadelphia WB Saunders. 1995.p.217-59.
2. Biogas K. Hypoxic ischemic brain injury: advancement
in the understanding of mechanisms and potential avenues for therapy. Curr
Opin Pediatr. 1999;11:223-31.
3. Vanucci RC, Perlman JM. Interventions for perinatal
hypoxic ischemic encephalopathy: relation to perinatal brain damage.
Pediatr Res.1990;27:317.
4. Fritz KI, Ashraf QM, Zubrow AB, Mishra OP,
Papadopoulous MD. Expression and phosphorylation of N-methyl d-aspartate
receptor subunits during graded hypoxia in cerebral cortex of newborn
piglets. Biol Neonate. 2004;85:128-37.
5. Saugstad OD. Mechanism of tissue injury by oxygen
radicals: implications for neonatal disease. Acta Pediatr. 1996;84:1-4.
6. Goldberg RN, Moscoso P, Bauer CR, Bloom FL, Curless
RG, Burki B. Use of barbiturate therapy in severe perinatal asphyxia. J
Pediatr. 1986;109:851-6.
7. Baughman LV, Hoffman W, Miltovich J, Albrecht
RF. Effects of phenobarbital on cerebral blood flow and metabolism in
young and aged rats. Anaesthesiology. 1986;65:500-5.
8. Vanucci RC, JM Perlman. Interventions for perinatal
hypoxic ischemic encephalopathy. Pediatrics. 1997;100: 1004.
9. Bewge JA, Aust SD. The thiobarbiturate assay.
Methods in Enzymology. 1978;52:306.
10. Hopkins J, Tudhope G. Glutathione peroxidise in
human red cells in health and disease. Br J Haematol. 1973;25: 563-7.
11. Misra HP, Fridovich I. The role of superoxide anion
in the autooxidation of epinephrine and a simple assay for superoxide
dismutase. J Biol Chem. 1972;247:3170-5.
12. Sarnat HB, Sarnat MS. Neonatal encephalopathy
following fetal distress: A clinical and electroencephalographic study.
Arch Neurol. 1976;33:696.
13. Singh D, Narang A, Kumar P, Majumdar S. Effect of
phenobarbital on free radicals in neonates with hypoxic ischemic
encephalopathy. J Perinatal Med. 2004;32:278-81.
14. Singh D, Kumar P, Narang A. A randomized controlled
trial of phenobarbital in neonates with hypoxic ischemic encephalopathy. J
Matern Fetal Neonatal Med. 2005;18: 391-5.
15. Hall RT, Hall FK, Daily DL. High dose phenobarbital
therapy in term newborn infants with severe perinatal asphyxia: a
randomized prospective study with three year follow up. J Pediatr.
1998;132:345-8.
16. Svenningsen NW, Blennow G, Lindroth M, Gaddlin PO,
Ahlstorm H. Brain oriented intensive care treatment in severe neonatal
asphyxia. Arch Dis Child. 1982;57:176-83.
17. Evans DJ, Levene MI, Tsakmakis M. Anticonvulsants
for preventing mortality and morbidity in full term neonates with
perinatal asphyxia. Cochrane Database Syst Rev. 2007:3:CD001240.
|
|
|
 |
|

