|
|
|
Indian Pediatr 2010;47: 687-693 |
 |
Hospital Based Nutrition Rehabilitation of
Severely Undernourished Children Using Energy Dense Local Foods |
|
Raja Sriswan Mamidi, Bharati Kulkarni, KV Radhakrishna and Veena Shatrugna
From the Clinical Division, National Institute of
Nutrition, Hyderabad, India.
Correspondence to: Dr Bharati Kulkarni, Scientist C,
National Institute of Nutrition (Indian Council of Medical Research),
Jamai Osmania PO, Hyderabad 500 007, India.
Email: [email protected]
Published online 2010, March 15.
PII: S097475590900379-1
|
|
Abstract
Objective: To examine the catch up growth in
severely wasted children using energy dense local foods at a hospital
based nutrition rehabilitation unit.
Design: Retrospective cohort.
Setting: In-patient ward at a tertiary care
government pediatric hospital in Hyderabad.
Patients: Children with severe malnutrition (n=309)
admitted to nutrition ward from January 2001 to December 2005.
Intervention: A diet based on energy dense local
foods along with multivitamin-multimineral supplements.
Main outcome measures: Catch up growth (g/kg/day)
during each week of hospital stay.
Results: Mean age of the children was 25
months (range 2-60). Their baseline weight for height (WHZ) Z
score was –4.1. Mean weight gain was moderate (5g/kg/day) and baseline
WHZ score had a significant negative relationship to the weight gain.
The prevalence of morbidities was high and the commonest morbidity was
fever. Weight gain was higher by almost 40% in the absence of
morbidities in any week.
Conclusions: The diet based on local energy dense
foods was found to be suitable for the nutrition rehabilitation of
severely malnourished children though the rate of weight gain was
moderate.
Key words: Catch up growth, Children, India, In-patient,
Nutrition rehabilitation, Protein energy malnutrition.
|
|
M
anagement of severely malnourished
children consists of an initial stabilization phase followed by a longer
rehabilitation phase, as per WHO guidelines(1). During rehabilitation,
rapid catch up growth in weight (>10g/kg/day) needs to be attained as it
facilitates early discharge and prevents secondary infections.
It is hard to find studies regarding catch up growth
during rehabilitation from hospitals in India due to centrality of
curative medical care, which takes most of the time of the doctors. In
addition, there is also heavy workload on the hospital staff and
standardized weighing scales are not available in many hospitals. As a
result, infrastructure for documentation of catch up growth in nutrition
rehabilitation with various diets (due to diverse food habits across the
country) is lacking.
We therefore examined the catch up growth in children
suffering from severe malnutrition using energy dense local foods.
Methods
The study was carried out at Niloufer Hospital,
Hyderabad where National Institute of Nutrition manages a nutrition ward
to provide nutrition support to the children who suffer from severe
mal-nutrition. Severely undernourished children who get admitted to the
hospital for various reasons are referred to the nutrition ward by the
clinicians attending to these patients after the initial treatment. This
includes necessary investigations, treatment of infections, correction of
electrolyte imbalance, and administration of broad-spectrum antibiotics
and this is carried out at other wards. The usual practice is to provide
potassium chloride if the child has symptoms suggestive of hypokalemia.
This initial phase of treatment usually requires a week and thereafter a
child is transferred to the nutrition ward for nutrition rehabilitation.
Criteria for admission to the nutrition ward is
presence of severe wasting (weight for height Z [WHZ]score <-3) or
nutritional edema or risk of severe wasting due to faulty feeding
practices(2). Once transferred to the ward, the child is examined
and necessary investigations including Mantoux test, hemoglobin
estimation, chest X-ray etc are carried out. Routine massive
vitamin A administration is done and iron is withheld for the first week
as per the WHO guidelines(1). Nursing staff is available round the clock
for supervised feeding. Broad spectrum antibiotics are administered to the
children who have not received antibiotics in other wards.
The diet provided to the children follows the WHO
recommendations regarding calories and proteins. However, it is modified
to include the local foods so that it is possible for families to maintain
it at home. This diet has been derived based on previous studies by
National Institute of Nutrition at this centre(3). The child is initially
put on a maintenance diet of about 100 kcal/kg/day, which is slowly
increased up to 170-220 kcal/kg/day. A typical diet of a child weighing
7kg consists of 350ml of milk (fortified with oil to increase the energy
density), 250g of khichdi (rice and dhal in 2:1 ratio with added
oil), 1-2 slices of bread, 2 eggs and a banana, which provides around 170
to 200 kcal/kg/day and 3 to 4 grams of protein/kg/day. Micronutrients are
provided in the form of multivitamin-multimineral syrups containing zinc
sulphate, nicotinamide, thiamine hydrochloride, riboflavin, pyridoxine,
copper sulphate, potassium iodide, selenium, cyanocobalamin, Vitamin A,
cholecalciferol and calcium. Folic acid is given regularly but iron is
withheld during the initial phase of rehabilitation. Iron supplementation
is started once the child starts gaining weight. Children are fed every 2
hours initially and once appetite improves, they are fed ad libitum.
Foods consumed by the children were recorded in a sub sample of 80
children who participated in another study(4).
All the children are weighed each morning on an
electronic weighing scale (SECA, Hamburg, Germany) to the nearest gram.
Length is measured in children
£2
years on day 1 to the nearest 0.1 cm with an infantometer made in-house at
our institute. For children aged >2 years, height is measured with an
anthropometric rod (SECA, UK). The doctor clinically examines the child
every morning and appropriate treatment is given. Any complaints narrated
by the caretaker and corroborated by the nurses are noted in the case
sheet. Temperature is recorded 8 hourly and charts are maintained.
For the present study, case sheets were obtained from
January 2001 to December 2005. Only those case sheets were selected where
the child stayed for ³7
days at the nutrition ward and the child was less than 5 years of age. The
following details were obtained from the case sheets - age, sex,
hemoglobin, initial clinical diagnosis indicating the cause of admission
to the hospital and presence or absence of edema, days of stay at other
ward and at nutrition ward, height on day 1, weight on day 1, day 7, day
14, day 21, day 28, day 35, and day of discharge. Day 1 represents the
first day at nutrition ward and week 1 represents the first week at
nutrition ward. Weight gain in each week was calculated in g/kg/day based
on WHO guidelines(1).
Rate of weight gain was also calculated for the entire
period of hospital stay (rate of weight gain for the entire duration of
the hospital stay).
Weight for height Z (WHZ) score, weight for age Z (WAZ)
score and height for age Z (HAZ) score were calculated at the beginning of
each week using WHO anthro (v2.0.4) software. Height was assumed to be the
same in all the weeks, as catch up growth in height in the first few weeks
of rehabilitation is usually not seen.
Morbidities were calculated for individual weeks. Days
of fever in that week were noted from temperature charts (presence of at
least one spike of temperature
³99
degree Fahrenheit on a given day was considered as a day of fever). Days
of diarrhea in that week were noted when doctor’s notes mentioned diarrhea
on that day. Days of other morbidities such as cough, vomiting and
respiratory distress etc were noted similarly. Total morbidity score was
calculated by adding up all the morbidity days in that week. For example,
if a child had fever and diarrhea on the same day, morbidity score was
calculated by adding up the two morbidities to give a score of 2 for that
day. If a child had fever for 3 days in a week without any other
morbidity, morbidity score was calculated as 3 for that week. If a child
had only fever for one day and fever with vomiting on another day in the
same week, morbidity score is calculated as 3 for that week.
Statistical analysis
All analyses were performed with the statistical
package for social science (SPSS) statistical software for windows, 11 (SPSS
Inc. USA). Mean and SD were calculated for continuous variables and
frequencies were calculated for categorical variables. One-way ANOVA with
Scheffe’s post-hoc tests for more than two groups and student’s t test
for two groups were calculated to see significant differences. Simple
regression analyses were used to assess the association of baseline WHZ
score with the rate of weight gain for the entire duration of the hospital
stay. Significance was tested at <0.05 level.
Results
Children admitted for nutrition rehabilitation belonged
to the lowest socioeconomic strata of the society. A total of 309
consecutive records were available for analysis. Out of these, 15 children
were readmitted to the ward. However, this number is likely to be an
underestimate of the true relapse rate, as children may not get admitted
to the same hospital or may not have been referred to the nutrition ward
from other wards.
Information on few variables was missing in the records
in some cases and available numbers are mentioned in parentheses in
tables. Mean age of the children was 25 months (range 2-60 months).
Baseline HAZ, WAZ and WHZ scores were –3.8 (range -11.1 to 1.1), -4.8
(range -8.1 to 1.0), and –4.1 (range -8.1 to –1.2), respectively. Among
them, 53% were boys, 18% had edema and 20% had severe anemia
(hemoglobin<7g/dL). The calorie and protein intakes calculated in a sub
sample during nutrition rehabilitation were (mean ± SD) 178 ± 54
kcal/kg/day and 4.1 ± 1.9 g/kg/day, respectively.
TABLE I
Weight Gain (g/kg/day) in Malnourished Children
| |
Edema present (n=56) |
Edema absent (n=253) |
|
Age (mo) |
32 ± 13 |
24 ± 15** |
|
Male:Female |
29:27 |
134:119 |
|
Duration of stay in nutrition ward (d) |
28 ± 20 (56) |
26
± 20 (253) |
|
Hemoglobin g/dL |
8.3 ± 2.3 (48) |
9.4
± 2.3* (194) |
|
Cause of admission % |
|
Diarrhea |
23 (13) |
26 (67) |
|
Pneumonia |
5 (3) |
12 (31) |
|
Others |
27 (15) |
21 (53) |
|
Tuberculosis |
20 (11) |
12 (31) |
|
Undernutrition |
25 (14) |
28 (71) |
|
Baseline WHZ score |
–4.3 ± 1.2 (53) |
–4.0 ± 1.3 (243) |
|
Baseline HAZ score |
–4.2 ± 1.6 (54) |
–3.7 ± 1.9 (246) |
|
Baseline WAZ score |
–5.0 ± 1.1 (56) |
–4.8 ± 1.3 (253) |
|
Rate of weight gain (g/kg/day) |
|
week 1 |
|
5.8
± 7.0 (252) |
|
week 2 |
7.0 ± 9.4 (43) |
5.7 ± 7.7 (169) |
|
week 3 |
4.1 ± 5.7 (31) |
5.5 ± 6.6 (123) |
|
week 4 |
4.8 ± 5.8 (22) |
5.2
± 5.7 (87) |
|
week 5 |
5.2 ± 7.8 (14) |
3.7 ± 6.8 (64) |
All the values are Mean ± SD; * P<0.01; ** P<0.001; WHZ-weight for height Z score; HAZ- height for age Z score;
WAZ -weight for age Z score.
|
Table I depicts the general characteristics and
rate of weight gain (g/kg/day) in children with and without edema.
Overall, the mean rate of weight gain calculated for the total duration of
the hospital stay in the entire sample was 5g/kg/day. Eight percent of the
children did not gain weight, 44% of the children had poor catch up growth
(<5g/kg/day), 35% of the children had moderate catch up growth
(5-10g/kg/day) and 12% had rapid catch-up growth (>10g/kd/day). Mean rate
of weight gain in each week appeared to decrease with increasing age (Table
II), but the difference was not statistically significant.
TABLE II
Weight Gain (g/kg/d) in Each Week in Relation to Baseline
|
Rate of weight gain |
| |
Week 1 |
Week 1 |
Week 1 |
Week 1 |
Week 1 |
Total |
|
Age |
|
≤12 months |
6.2 ± 8.91 (80) |
4.9 ± 9.1 (53) |
7.2 ± 6.9 (41) |
7.0 ± 6.1 (32) |
4.4 ± 8.5 (23) |
5.3 ± 5.6 (80) |
|
13-24 months |
6.5 ± 6.9 (80) |
7.9 ± 7.4 (51) |
4.9 ± 6.2 (36) |
2.9 ± 5.4 (24) |
2.8 ± 5.7 (21) |
6.1 ± 5.2 (80) |
|
25-36 months |
5.6 ± 5.5 (56) |
4.9 ± 6.8 (38) |
4.7 ± 7.2 (31) |
6.8 ± 5.1 (18) |
1.7 ± 7.1 (9) |
4.8 ± 4.1 (56) |
|
37-48 months |
3.8 ± 3.6 (19) |
5.4 ± 6.1 (12) |
5.1 ± 5.4 (6) |
1.8 ± 5.1 (5) |
8.8 ± 4.7 (4) |
3.7 ± 3.1 (19) |
|
49-60 months |
4.3 ± 4.0 (18) |
2.8 ± 5.5 (15) |
3.6 ± 3.3 (9) |
3.4 ± 4.0 (8) |
3.5 ± 3.0 (7) |
3.4 ± 2.5 (18) |
|
Baseline WHZ score |
|
≤-3 |
6.6 ± 7.2 (183)** |
6.9 ± 7.7(110) * |
6.0 ± 6.2 (70) |
5.5 ± 5.9 (44) |
5.0 ± 6.9 (25) |
5.9±4.7(183) ** |
|
>-3 |
3.3 ± 5.5 (59) |
3.9 ± 6.8 (51) |
4.8 ± 6.9 (50) |
4.8 ± 5.6 (42) |
2.6 ± 6.6 (37) |
3.1 ± 4.4 (59) |
|
Cause of admission |
|
Diarrhea |
6.8 ± 7.6 (66) |
6.3 ± 9.3 (38) |
8.2 ± 6.6 (29) |
6.1 ± 5.3 (21) |
4.2 ± 6.0 (12) |
6.3 ± 5.7 (67) |
|
Pneumonia |
5.4 ± 8.2 (31) |
3.8 ± 7.0 (26) |
2.7 ± 8.1 (15) |
7.5 ± 7.2 (9) |
6.1 ± 6.2 (8) |
3.9 ± 3.2 (31) |
|
Tuberculosis |
6.4 ± 7.8 (31) |
8.4 ± 7.9 (27) |
5.4 ± 7.4 (24) |
2.9 ± 6.0 (21) |
1.8 ± 7.1 (16) |
5.8 ± 5.5 (31) |
|
Others |
4.6 ± 7.1 (53) |
5.6 ± 7.3 (31) |
3.9 ± 5.0 (23) |
5.0 ± 4.8 (17) |
3.3 ± 8.3 (14) |
4.3 ± 4.8 (53) |
|
No infection |
5.7 ± 5.2 (71) |
4.5 ± 6.5 (47) |
5.5 ± 5.2 (32) |
5.7 ± 5.7 (19) |
4.3 ± 6.0 (14) |
5.0 ± 4.0 (71) |
|
Morbidity |
|
Days of fever without other morbidities |
|
0 |
7.1 ± 6.6a (90)** |
7.4±6.0a (73)** |
7.0±5.3a(48)** |
7.8 ± 5.0a (42)** |
6.6 ± 5.1a (26)** |
|
|
1-2 |
7.8 ± 6.3a (87) |
7.0 ± 7.0a (41) |
7.2 ± 6.0a (35) |
5.3 ± 5.1a (20) |
6.6 ± 6.2a (16) |
|
|
>2 |
2.3 ± 6.6b (29) |
1.2 ± 7.7b (20) |
2.5 ± 6.3b (13) |
-0.4 ± 3.4b (14) |
-0.3 ± 5.6b (11) |
|
|
Days of diarrhea without other morbidities |
|
0 |
7.1 ± 6.6 (90) |
7.4 ± 6.0 (73) |
7.0 ± 5.3 (48) |
7.8 ± 5.0 (42) |
6.6 ± 5.1 (26) |
|
|
1-2 |
4.5 ± 5.2 (10) |
5.8 ± 8.9 (11) |
3.2 ± 5.8 (9) |
|
0 (1) |
|
|
>2 |
|
-1.6 ± 2.2 (2) |
|
|
|
|
|
Total morbidity score |
|
0 |
7.1 ± 6.6a (90)** |
7.4±6.0a (73)** |
7.0±5.3a(48)** |
7.8 ± 5.0a (42)** |
6.6 ± 5.1a (26)** |
|
|
1-2 |
6.9 ± 6.2a (110) |
6.4 ± 7.8a (61) |
6.4 ± 6.4a (51) |
5.2 ± 4.7a (23) |
4.5 ± 6.2a (23) |
|
|
>2 |
1.2 ± 7.4b (51) |
1.0 ± 9.1b (33) |
0.6 ± 7.7b (22) |
-0.5 ± 3.8b (20) |
-2.6 ± 6.3b (15) |
|
|
All the values are Mean
± SD; n in brackets. ANOVA (more than 2 groups), and student’s t test
(two groups) are calculated between rows for each variable in each
week; * P<0.05, ** P<0.001. |
Most of the children had 1-2 days of fever during
admission and about 10-12% children had diarrhea. Longer duration of fever
worsened the rate of weight gain in each week. Similarly, higher morbidity
score was associated with lower rate of weight gain.
In simple linear regression analysis with rate of
weight gain for the total duration of the hospital stay as dependent
variable and baseline WHZ score on day 1 as an independent variable,
baseline WHZ score was significant determinant (P<0.001) of weight
gain explaining 12 % of the variation in total weight gain (Fig.
1).
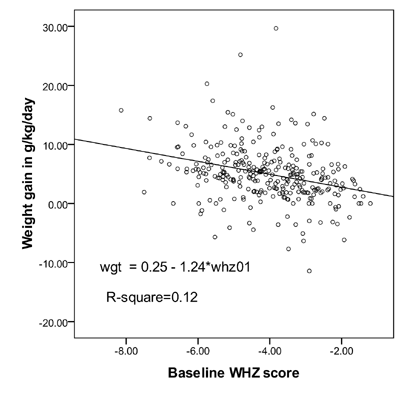 |
|
Fig. 1 Linear regression plot with (weight
for height Z) WHZ score as independent variable and total weight
gain (wgt) as dependent variable in children without edema.
|
Discussion
The study provides a database on catch up growth in
weight during nutrition rehabilitation of severely undernourished children
when treated with energy dense local foods. The mean rate of weight gain
at our center was 5g/kg/day (Table 1). Despite staying in
the ward for 5 weeks, mean WHZ scores of 40% of children were <-3 due to
severity of baseline wasting and moderate rates of catch up growth. Rapid
catch up growth of >10g/kg/day in hospital-based rehabilitation was seen
in centers from Jamaica and Bangladesh(5-7). Studies reporting catch up
growth during hospital based nutrition rehabilitation in India are not
available for comparison.
WHO recommends caloric intake of 170-220 kcal/kg/day
for rapid catch up growth during nutrition rehabilitation. The mean
calorie and protein intakes at the ward calculated in a sub sample of
children were about 180 kcal/kg/day and 4 g/kg/day, respectively, which
may be considered adequate. Zinc was found to be a limiting factor despite
high calorie diets in a study from Bangladesh(8). However, zinc and other
micronutrients are routinely supplemented at our center. Milk based diets
were found to be superior to plant based diets for catch up growth(9,10)
and regenerating serum albumin(11,12). The diet at our center can be
considered as a mixed diet with average milk intakes of about 350mL per
day (range 50mL to 850mL). Rapid catch growth was possible with local
staples consisting of meat, fish, vegetables and palm oil in a study from
Nigeria(13). With the advent of ready to use therapeutic food (RUTF), much
more rapid catch up growth (15g/kg/day) was observed as compared to the
WHO recommended milk based diet (F-100) in a therapeutic feeding center in
Senegal(14).
Although the calorie-protein intakes of the children at
our center appear to be adequate, the intakes of potassium and magnesium
may be inadequate. WHO guidelines advocate supplemental potassium and
magnesium for at least 2 weeks for all severely malnourished children. At
our center, these supplements were provided when the child had clinical
symptoms suggestive of hypokalemia. These supplements were not provided
routinely at the ward because magnesium is not commonly available as an
oral supplement, and the children received a variety of foods which were
rich sources of potassium and magnesium. However, the intakes of potassium
and magnesium in these children were less than the WHO recommendation and
this may have contributed to the low rates of weight gain. Better
adherence to the WHO guidelines may help improving the rate of weight gain
during nutrition rehabilitation of severely malnourished children.
The high prevalence of fever and presence of other
morbidities is not entirely unexpected, as mal-nourished children are
susceptible to infections due to poor immune status(15-17). Therefore,
despite efforts to prevent infections, the morbidities such as fever were
high. In this study, weight gain was 7g/kg/day when there was no
morbidity, which was 40 % higher than the average weight gain of
5g/kg/day.
Apart from the caloric intake, the severity of wasting
is known to influence the rate of weight gain(18). Interestingly in the
regression plot of our study, weight gain decelerated to zero when the
child’s baseline WHZ score approached the median (Fig. 1).
With increasing age, there was a decrease in weight gain though
statistically not significant. But such pattern is expected since growth
rates are much higher in the first two years of life compared to the next
three years.
Rapid catch up growth is required to hasten the rate of
recovery and provide beds to other ailing children(19). In the present
study, which included children who stayed for more than a week, only one
out of five stayed for
³5
weeks. Their parents were daily-wage workers and were unable to stay for
longer duration.
All the limitations of a retrospective study are
applicable to this study but since our center is a research unit, the
staff is well trained and experienced and records are well maintained.
Moreover, as this study included data collected for 5 years, sample size
was large and it was possible to carry out sub-group analyses.
Management of severely undernourished children as per
the WHO guidelines aims at achieving weight gain of >10g/kg/day and it
needs to be examined whether a better adherence to WHO guidelines would
improve the rate of weight gain.
Acknowledgments
Dr B Sesikeran, Director, National Institute of
Nutrition, Hyderabad, India for administrative support, and Ms Pramodini,
Ms Rajkumari and other nurses for preserving the records.
Contributors: All the authors conceived and
designed the study. RSM analyzed the data and wrote the paper. BK, KVR and
VS guided data analysis and interpretation of data, and helped editing the
paper. All the authors were involved in providing clinical care to the
children involved in the study. All the authors approved the final
manuscript.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• Information on catch up
growth during nutrition rehabilitation of severely undernourished
children reported from other countries is largely based on
milk-based diets.
What This Study Adds?
• Moderate catch up growth can be achieved
in severely undernourished children treated with energy dense local
foods in a hospital setting.
|
References
1. World Health Organization. Guidelines for the
inpatient treatment of severely malnourished children. Geneva: WHO; 2003.
2. World Health Organization. Multicentre Growth
Reference Study Group: WHO Child Growth Standards: Length/height-for-age,
weight-for-age, weight-for-length, weight-for-height and body mass
index-for-age: Methods and development. Geneva: WHO; 2006.
3. Reddy V, Bhaskaram P. Treatment of severe protein
energy malnutrition. Indian Pediatr 1982; 19: 243-248.
4. Radhakrishna KV, Kulkarni B, Balakrishna N,
Hemalatha R, Chandrakala OA, Shatrugna V. Composition of weight gain
during nutrition rehabilitation of severely under nourished children in a
hospital based study from India. Asia Pac J Clin Nutr 2010; 19: 8-13.
5. Khanum S, Ashworth A, Huttly SR. Controlled trial of
three approaches to the treatment of severe malnutrition. Lancet 1994;
344: 1728-1732.
6. Ashworth A. Growth rates in children recovering from
protein-calorie malnutrition. Br J Nutr 1969; 23: 835-845.
7. Iqbal HM, Dodd NS, Tahmeed A, Mothabbir MG, Jamil
Kazi M, Baitun N, et al. Experience in managing severe malnutrition
in a government tertiary treatment facility in Bangladesh. J Health Popul
Nutr 2009; 27: 72-79.
8. Simmer K, Khanum S, Carlsson L, Thompson RP.
Nutritional rehabilitation in Bangladesh—the importance of zinc. Am J Clin
Nutr 1988; 47: 1036-1040.
9. Graham GG, Baertl JM, Cordano A. Studies in
infantile malnutrition. V. The effect of dietary protein source on serum
proteins. Am J Clin Nutr 1966; 18: 16-19.
10. Pereira SM, Begum A. The manifestations and
management of severe protein-calorie malnutrition (kwashiorkor). World Rev
Nutr Diet 1974; 19: 1-50.
11. Srikantia SG, Gopalan C. Clinical trials with
vegetable protein foods in kwashiorkor. Indian J Med Res 1960; 48:
637-644.
12. Venkatachalam PS, Srikantia SG, Geeta Mehta,
Gopalan C. Treatment of Nutritional Edema Syndrome (kwashiorkor) with
vegetable protein diets. Indian J Med Res 1956; 44: 539-545.
13. Smith IF, Taiwo O, Golden MHN. Plasma somatomedin
in Nigerian malnourished children fed a vegetable protein rehabilitation
diet. Eur J Clin Nutr 1989; 43: 705-713.
14. Diop el HI, Dossou NI, Ndour MM, Briend A, Wade S.
Comparison of the efficacy of a solid ready to use food and a liquid
milk-based diet for the rehabilitation of severely malnourished children:
a randomized trial. Am J Clin Nutr 2003; 78: 302-307.
15. Walker SP, Grantham-McGregor SM, Powell CA, Himes
JH, Simeon DT. Morbidity and the growth of stunted and nonstunted
children, and the effect of supplementation. Am J Clin Nutr 1992; 56:
504-510.
16. Becker S, Black RE, Brown KH. Relative effects of
diarrhea, fever and dietary energy intake on weight gain in rural
Bangladeshi children. Am J Clin Nutr 1991; 53: 1499-1503.
17. Bhaskaram P. Micronutrient malnutrition, infection,
and immunity: an overview. Nutr Rev 2002; 60: S40-45.
18. Ashworth A, Millward DJ. Catch-up growth in
children. Nutr Rev 1986; 44: 157-163.
19. Waterlow JC. Protein-Energy Malnutrition. In: Waterlow JC,
ed. London: Edward Arnold; 1992. p. 165-186.
|
|
|
 |
|

