|
|
|
Indian Pediatr 2009;46: 675-680 |
 |
Serum Copper and Zinc Levels in Mothers and
Cord Blood of their Newborn Infants with Neural Tube Defects:
A Case-control Study |
|
Dost Zeyrek, Mustafa Soran, Alpay Cakmak, Abdurrahim Kocyigit* and Akin
Iscan
From the Departments of Pediatrics and *Biochemistry,
Harran University School of Medicine, Sanliurfa, Turkey.
Correspondence to: Dr Dost Zeyrek, Department of
Pediatrics, Harran University School of Medicine, TR-63100,
Sanliurfa, Turkey. E-mail:
[email protected]
Manuscript received: February 28, 2008;
Initial review completed: May 14, 2008; Revision accepted: October 3,
2008.
|
|
Abstract
Objectives: To measure the cord blood and
maternal serum levels of folic acid, vitamin B12, zinc, copper, selenium
and lead in infants born with neural tube defect (NTD), and to examine a
possible relationship between the nutriture of these micronutrients and
occurrence of neural tube defect.
Design: Case-control study.
Methods: Maternal serum and cord blood samples
were obtained at delivery from 70 healthy mothers and 74 mothers who had
a newborn with NTD.
Results: The mean (± SD) maternal serum zinc
level in the NTD group was significantly lower than that of the control
group (835.6 µg/L ±333.8 µg/L vs. 1035.7 µg/L ± 299.8 µg/L, P=0.004,
respectively). The mean maternal and cord serum copper levels in the NTD
group were significantly higher when compared to the control group
(2831.1 µg/L ± 1017 µg/L vs. 2402 µg/L ± 744.2 µg/L; P=0.03;
and 789.8 µg/L vs 517.2 µg/L, P<0.001, respectively).
There was a negative correlation between the cord levels of folic acid
and copper in the NTD group with the respective maternal serum levels (r=-0.289;
P=0.018).
Conclusions: High maternal serum levels of copper
and lower level of zinc during pregnancy associated with NTD in newborn.
Keywords: Copper, Folic acid, Micronutrients, Neural tube
defects, Zinc.
|
|
N
eural tube defects (NTD) are one of
the most common forms of human congenital malformations(1). NTD occur in
1-6.5 per 1,000 births, with marked geographic and ethnic variations(2,3).
Comparatively, the incidence of NTD at 9.5/1,000 births in our region is
somewhat high(4). The etiology of NTD is multifactorial involving
nutritional deficiencies, genetic predisposition and environmental
factors(5). Nutritional factors appear to be an important contributor to
the etiology of NTD. Although it is known that folic acid deficiency has a
definite place in the etiology, supplementation and fortification with
folic acid have not eliminated all NTD.
The role of vitamin B 12
and other trace elements such as copper (Cu) and zinc (Zn) is still
uncertain and there is a limited amount of published literature on this
topic. Cu is an important component of proteins essential for neural
function. The role of copper in the development of NTD is plausible
because of its participation in oxidative stress(6-9). Also, zinc is an
essential nutrient for normal cellular growth and differentiation in all
species and may be especially necessary for closure of the human neural
tube. We investigated the cord blood and maternal serum levels of folic
acid, vitamin B12, Zn, Cu, selenium (Se) and lead (Pb) in infants born
with NTD to examine a possible relationship between the nutriture of these
micronutrients and occurrence of NTD.
Methods
The study was carried out at Sanliurfa Maternity
Hospital and the Maternity and Obstetrics Clinic of Harran University
Medical School. Seventy-four newborns (gestational age
ł20
weeks) with NTD (excluding spina bifida occulta) formed the cases and
seventy healthy infants born in the same period and from the similar
socioeconomic group were used as controls. Babies having possibility of
infection and a positive C-Reactive Protein (CRP) level were excluded from
the study. Informed consent was obtained from the families and approval
was obtained from the local Ethics Committee. A record was taken of the
age of the mother, consanguinity of parents. medical history, the number
of pregnancies, abortions, history of previous NTD, use of medication,
smoking, proximity to radiation during pregnancy, and results of
intrauterine ultrasonography (USG). The gestation period, birthweight and
length were recorded.
Umbilical cord blood samples and maternal venous
samples were collected within 30 minutes after the birth and serum was
separated. After immediate centrifugation, serum was transferred to
deionized tubes, stored and frozen at –80şC until determination of Se, Pb,
Zn and Cu concentrations was done.
Serum Se and Pb concentrations were determined by a
SpectrAA 250 Plus Zeeman Atomic Absorption A spectrometer with a graphite
furnace GTA-97 (Varian, Australia) with deuterium background correction
using standard method(10,11). Serum Zn and Cu levels were determined by
Atomic Absorption Spectrometer (Varian Spectr AA 250 Plus, Australia).
Serum samples were diluted (1:5) with ultra deionized water. Cu and Zn
values were expressed in mg/L. Serum iron concentration was determined by
colorimetric method with a commercial kit (Boehringer Mannheim, Germany)
using an automatic analyzer (Hitachi 911, Boehringer Mannheim, Germany).
Serum folic acid and vitamin B 12
levels were determined by commercial kits (Roche Diagnostic, Germany) with
an automatic hormone analyzer (Elecsys 2010, Germany). CRP levels were
determined by an immunoturbidimetric assay.
Statistical analysis of the data was performed with
SPSS Version 11.0. Differences in demographic data between the study group
and control group were compared by Chi-square test (or Fisher’s exact
test, if the predicted number of
subjects in any category was less than five). Median levels of trace
elements were compared between the study group and the control group women
and their newborns with the use of Student’s t-test and
Mann-Whitney U test according to distribution normality. Presence of NTD
was considered as the dependent factor in multivariate logistics
regression analysis. Independent factors included in the analysis as
dichotomous variables were parity, history of a previous NTD, history of
abortions, multivitamin use, maternal smoking, and infection in pregnancy.
The correlations within the groups between folic acid, vitamin B12 and
trace elements were assesed by Pearson’s rank correlations (rp).
Statistical significance was defined for P values of less than 0.05.
Results
The parental characteristics of patients and control
subjects are shown in Table I. Of the infants with NTD, 44
(59.5%) had anencephaly, 3 (4%) had encephalocele, and 27 (36.5%) were
diagnosed with spina bifida. A prenatal diagnosis had been made on 43
(58.1%) of the babies with NTD.
TABLE I
Baseline Characteristics of the Study and Control Groups
| |
Case (N=74) |
Control
(N=70) |
OR (95%CI) |
P value |
| Age
(years)* |
28.8±7.3 (17-49) |
25.8±5.8 (17-39) |
|
0.03 |
|
Maternal age groups |
|
<20 |
6 |
8 |
0.7 (0.2-2.3) |
0.50 |
|
20-35 |
53 |
58 |
0.5 (0.2-1.3) |
0.10 |
|
>35 |
15 |
4 |
4.1 (1.3-13.3) |
0.01 |
| Newborn
gender (M/F) |
34/40 |
26/44 |
1.4 (0.7-3.0) |
0.28 |
|
Gestational age (weeks)* |
34.2±6.2 (20-41) |
39.3±0.8 (37-41) |
|
<0.001 |
| Birth
order* |
3.9±2.8 (1-13) |
3.3±2.3 (1-10) |
|
0.22 |
| History
of previous NTD, n (%) |
5 (6.8) |
1 (1.4) |
5 (0.6-43.9) |
0.11# |
| History
of abortion, n (%) |
14 (18.9) |
8 (11.4) |
1.8 (0.7-5.1) |
0.21 |
|
Multivitamin use, n (%) |
6 (8.1) |
2 (2.8) |
3 (0.5-22.4) |
0.16 |
| Maternal
smoking, n (%) |
8(10.8) |
14(20) |
0.5 (0.2-1.4) |
0.12 |
| Infection
in pregnancy, n (%) |
4 (5.4) |
3(4.3) |
1.3 (0.3-5.9) |
0.53# |
|
*The data are given as mean±SD (range); # Fisher’s exact test. |
The mean (± SD) Zn level of the mothers who gave birth
to infants with NTDs was significantly lower when compared to the control
group (835.6 ±333.8 vs. 1035.7±299.8; P=0.004). However, the
mean maternal and cord serum Cu levels in the NTD group were significantly
higher when compared to the control group (2831.1±1017 vs.
2402±744.2, P=0.03 and 789.8 vs. 517.2, P<0.001,
respectively). In comparison with the control group, the ratio of Cu/Zn in
the mothers of the babies with NTD was found to be significantly higher
(3.8±2.1 vs. 2.6±1.2, P=0.001) (Table II). A
statistically significant nega-tive relationship was determined between
the levels of Cu and folic acid in the cord blood of the new-borns with
NTDs and their mothers (r=–0.401; P=0.001 and r=
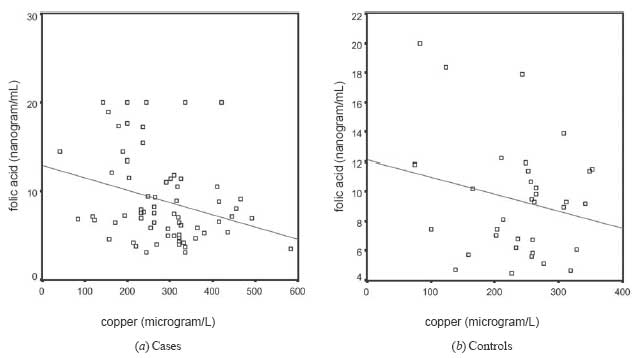
–0.289; P=0.018, respectively) (Fig. 1 and
Fig. 2).
TABLE II
Serum Levels of Folic Acid, B12 and Trace Elements in Mothers and Newborns (Mean±SD)
| |
Mothers |
Newborns |
|
Element |
NTD (N=74) |
Control (N=70) |
P value |
NTD (N=74) |
Control (N=70) |
P value |
| Folic acid (ng/mL) |
9.0±4.9 |
9.4±3.9 |
0.66 |
18.0±7.0 |
15.9±3.3 |
0.10 |
| Vitamin B12 (pg/mL) |
217.8±125.5 |
261.6±154.3 |
0.12 |
309.1±182.4 |
407.3±391.7 |
0.10 |
| Copper (µg/L) |
2831.1±1017.0 |
2402±744.2 |
0.03 |
789.8 |
517.2 |
<0.001 |
| Zinc (µg/L) |
835.6±333.8 |
1035.7±299.8 |
0.004 |
1390.7±504.4 |
1294.1±345.7 |
0.33 |
| Selenium (µg/L) |
46.8±26.4 |
47.6±20.6 |
0.87 |
42.2±21.9 |
39.9±20.0 |
0.60 |
| Lead (µg/L) |
155.0±150.4 |
125.4±126.5 |
0.35 |
182.2±177.8 |
164.5±161.0 |
0.63 |
| Iron (µg/dL) |
63.1±15.9 |
66.2±19.0 |
0.61 |
82.3±19.0 |
87.0±20.7 |
0.50 |
| Copper/Zinc |
3.8±2.1 |
2.6±1.2 |
0.001 |
1.0±1.1 |
0.7±1.3 |
0.32 |
 |
|
Fig. 1
The levels of serum copper and serum folic acid in the mothers of
children with NTD and the controls.
|
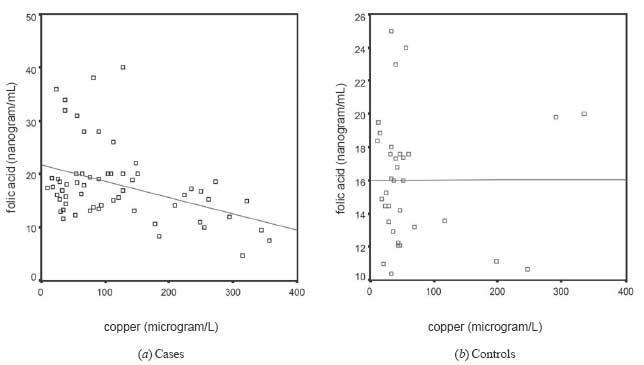 |
|
Fig. 2 The levels
of serum copper and serum folic acid in cord blood of the infants
with NTD and the controls. |
Discussion
In our study, both the newborn infants with NTDs and
their mothers had high levels of serum Cu and low levels of Zn when
compared to the control group. However, the levels of folic acid, vitamin
B 12, Se, Pb and Fe were not
significantly different between the two groups.
Our study results showed that in comparison with the
control group, the mothers who had given birth to babies with NTD had high
levels of serum copper and low levels of zinc. This is in line with the
findings of Cengiz, et al.(7). This difference between the two
groups may be related to the levels of copper and zinc in the environment
and their nutrition. It is known that serum levels of copper and zinc
could be high in mothers from an area where the soil has high levels of
these elements(12). However, the role played in the development of NTD by
the level of Cu in the water supply is controversial(8). Copper and zinc
levels are closely related to nutrition. The poor socioeconomic conditions
of Sanliurfa and the consumption of yeast-free bread are seen as the
reasons for the widespread lack of Zn(13). Furthermore, there are
important interactions between trace elements and vitamins at the level of
intestinal absorption. When there is a decrease in Zn, the increase in Cu
absorption is the reason for the increased level of serum copper. Zn-Cu
interaction during intestinal absorption may be the answer for the
relative increase in Cu in patients with NTDs in our series. The level of
serum copper increases and that of zinc decreases in inflammation(14). NTD
itself gives rise to inflammation explaining the pathology of serum copper
and zinc levels in our study. An earlier study has shown that the level of
serum copper in pathological pregnancies was significantly higher in
comparison to normal pregnancies(12).
Although in normal pregnancies, possibly because of the
placental transfer to the baby, serum zinc level decreases(15), in our
study the serum level of Zn in the mothers who had infants with NTD was
significantly lower in comparison to the control group. This difference in
serum zinc levels between the cases and controls is unlikely to be related
to the difference in gestational age seen in our study. Previous studies
have not detected any difference in the serum zinc level of mothers of
preterm and full-term babies(16). It appears that the low level of serum
Zn in the mother affects the development of NTD. Furthermore, high intake
of zinc may provide protection against Cu toxicity by preventing excess Cu
uptake. Zinc also removes Cu from its binding site, where it may cause
free radical formation(17). Zn may also play a role by influencing copper
metabolism in the development of NTD. There might also be a relationship
between genetic and metabolic processes involving the pathology of the
serum copper and zinc levels of the mothers of babies with NTD.
Furthermore, we established a negative correlation between the high level
of serum Cu and the level of serum folic acid in the mothers of infants
with NTD. It is plausible that a high level of Cu plays a role in etiology
of NTD by having a negative effect on the folic acid nutriture.
In conclusion, as the etiology of NTD is thought to be
multifactorial, a lack or an excess of trace elements and the interactions
between vitamins and trace elements may play a role in its development.
Our results indicate that Zn supplements and attention to the high serum
copper level may be important in the prevention of NTD. Large scale
prenatal zinc supplementation trials are therefore recommended to further
confirm this association.
Contributors: All authors contributed to the study
design, collection of data, analysis and drafting the manuscript.
Funding: None.
Competing interest: None stated.
|
What is Already Known?
• Nutritional factors are important in the
pathogenesis of neural tube defects.
What this study adds?
• Mothers giving birth to infants with neural
tube defects have high serum levels of copper and low serum levels
of zinc. There is a negative correlation between the high level of
serum copper and the level of serum folic acid in the mothers of
infants with neural tube defects.
|
References
1. Frey L, Hauser WA. Epidemiology of neural tube
defects. Epilepsia 2003; 44 (suppl. 3): 4-13.
2. Aqrabawi HE. Incidence of neural tube defects among
neonates at King Hussein Medical Centre, Jordan.East Mediterr Health J
2005; 11: 819-823.
3. Feuchtbaum LB, Currier RJ, Riggle S, Roberson M,
Lorey FW, Cunningham GC. Neural tube defect prevalence in California
(1990–1994): Eliciting patterns by type of defect and maternal
race/ethnicity. Genet Testing 1999; 3: 265-272.
4. Zeyrek D, Iscan A, Sevinc E, Yildiz F, Mil Z,
Karazeybek H. The prevalence of neural tube defects in Sanliurfa. New Med
2004; 21: 252-255 (in Turkish).
5. Rengasamy P. Etiology, pathogenesis and prevention
of neural tube defects. Congenit Anom 2006; 46: 55-67.
6. O’Shea KS, Kaufman MH. Influence of copper on the
early post-implantation mouse embryo: an in vivo and in vitro study. 1979;
186: 297-308.
7. Cengiz B, Soylemez F, Ozturk E, Cavdar AO. Serum
zinc, selenium, copper, and lead levels in women with second-trimester
induced abortion resulting from neural tube defects: a preliminary study.
Biol Trace Elem Res 2004; 97:225-235.
8. Morton MS, Elwood PC, Abernethy M. Trace elements in
water and congenital malformations of the central nervous system in South
Wales. Br J Prev Soc Med 1976; 30: 36-39.
9. Burkitt MJ. A critical overview of the chemistry of
copper-dependent low density lipoprotein oxidation: roles of lipid
hydroperoxides, alpha-tocopherol, thiols and ceruloplasmin. Arch Biochem
Biophys 2001; 394: 117-135.
10. Milde D, Novak O, Stuzka V, Vyslouzil K, Machacek
J. Serum levels of selenium, manganese, copper, and iron in colorectal
cancer patients. Biol Trace Elem Res 2001; 79: 107-114.
11. Fernandez FJ. Micromethod for lead determination in
whole blood by atomic absorption, with use of graphite furnace. Clin Chem
1975; 21: 558-561.
12. Alebic-Juretic A, Frkovic A. Plasma copper
concentrations in pathological pregnancies. J Trace Elem Med Biol 2005;
19: 191-194.
13. Kocyigit A, Koc A, Erel O, Vural H, Atas A. The
hematological parameters and serum levels of some trace elements in
children who eat bread made without or with yeast. New J Med 1998; 15:
206-210 (in Turkish).
14. Shenkin A. Trace elements and inflammatory
response: implications for nutritional support. Nutrition 1995; 11 (1
Suppl):100-105.
15. Tamura T, Goldenberg RL, Johnston KE, DuBard M.
Maternal plasma zinc concentrations and preg-nancy outcome. Am J Clin Nutr
2000; 71: 109-113.
16. Bahl L, Chaudhuri LS, Pathak RM. Study of serum
zinc in neonates and their mothers in Shimla hills (Himachal Pradesh).
Indian J Pediatr 1994; 61: 571-575.
17. Powell SR. The antioxidant properties of zinc. J Nutr 2000; 130:
1447S-1454S.
|
|
|
 |
|

