|
|
|
Indian Pediatr 2021;58:370-376 |
 |
Steroids for the
Management of Neonates With Meconium Aspiration Syndrome: A
Systematic Review and Meta-analysis
|
|
Telford Yeung, 1,2 Bonny Jasani1,2
and Prakesh S Shah1,3
From 1Division of Neonatology, University of Toronto
Department of Pediatrics, 2Hospital for Sick Children, 3Department
of Pediatrics, Mount Sinai Hospital, Toronto, Canada.
Correspondence to: Dr Telford Yeung, Department of Pediatrics, Mount
Sinai Hospital, 600 University Avenue, Toronto, ON, M5G 1X5, Canada.
Email:
[email protected]
|
Background: Steroids are a potential treatment
for pulmonary inflammation in meconium aspiration syndrome (MAS).
Objective: To assess the efficacy and safety of steroids for the
management of neonates with MAS. Design: Systematic review and
meta-analysis of randomized controlled trials (RCT). Data sources and
selection criteria: A systematic search of PubMed, Embase,
Cochrane, and CINAHL was performed from database inception to May 2020
for trials assessing the efficacy of steroids (inhaled/systemic or both)
in neonates with MAS. The primary outcome was in-hospital mortality,
with secondary outcomes being length of hospital stay and duration of
oxygen support. Results: Nine RCTs (758 neonates) were included.
Overall, steroids did not decrease in-hospital mortality (RR: 0.59; 95%
CI 0.28 to 1.23; I2 = 0%; GRADE: low) nor had any effect on the
secondary outcomes. Conclusion: There is low quality of evidence
that the administration of steroids is not associated with a reduction
in mortality in infants with MAS. Further well-designed studies with low
bias are needed to draw conclusions.
Keywords: Dexamethasone, Meconium-stained amniotic fluid,
Surfactant, Outcome.
|
|
M
econium aspiration syndrome (MAS) occurs
in newborns born through meconium stained amniotic fluid [1].
While in the Vermont Oxford Network, MAS accounted for 1-2% of
all NICU admissions with a mortality rate of 2-3% [2]; in India,
MAS was the second leading cause of neonatal admissions with
mortality ranging between 13% and 32% [3], thus having a
substantial impact on hospital expenditure [4]. The current
standard of care is supportive therapy with oxygenation and
mechanical ventilation [5], with antibiotics and surfactant
being common adjunct therapies [5]. For severe cases of MAS,
additional interventions include pulmonary vasodilators like
inhaled nitric oxide (iNO), vasoactive drug infusions, and
extracorporeal membrane oxygenation (ECMO) [5]. Neither
antibiotics nor surfactant have shown satisfactory outcomes with
respect to mortality in this condition. [6, 7].
The role of steroids in MAS was reported in a
systematic review by Cochrane group, in 2003, concluding no
benefit of steroids [8-10]. However, this review was limited by
a small sample size (85 patients) receiving a suboptimal dose
regimen [9,10]. Recent animal studies [11,12] have renewed
interest in steroids resulting in several small, single centred
randomized controlled trials (RCTs) from resource limited
countries where the availability of iNO and ECMO are scarce
[13-19]. Therefore, our objective was to systematically review
and meta-analyze the efficacy and safety of steroid therapy
compared to placebo for infants with MAS. We also intended to
assess the type and mode of administration of steroids [13-19].
METHODS
We followed guidelines from the Cochrane
Neonatal Review Group [20] for conducting a systematic review
and the Preferred Reporting Items for Systematic Reviews and
Meta-analyses (PRISMA) guidelines [21] for reporting the results
of systematic reviews with meta-analysis. The study was exempted
from ethics review.
Search strategy: TY and BJ conducted
independent searches of the medical databases namely, Medline,
Embase, and Cumulative Index of Nursing and Allied Health
Literature (CINAHL) databases as well as Cochrane Central
Register of Controlled Trials (CENTRAL), without any language
restriction, published before May 11, 2020. We also searched
first 200 hits of Google Scholar for articles that may not have
been indexed in the standard medical databases. The details of
the search terms used for the databases and the search output
have been shown in Supp. Table I.
Search eligibility: Randomized controlled
trials studying the efficacy or safety of steroids in newborns
with MAS were included. Cross-over studies, systematic reviews
and animal-based studies were excluded. Newborns fulfilling the
criteria of late preterm (34+0 to 36+6 weeks gestation), term or
post term infants were included. Studies where MAS was diagnosed
either by direct aspiration of meconium from below the larynx or
respiratory distress within few hours after birth and
radiographic features of an aspiration syndrome, were included.
The intervention studied was administration of steroids (either
inhaled or systemic) in any dose, given within 36 hours of
birth, for any duration, for the management of infants diagnosed
with MAS compared to no intervention or placebo. The primary
outcome for this study was in-hospital mortality. The secondary
outcomes were length of hospital stay, duration of oxygen
therapy, need for and duration of mechanical ventilation,
steroid associated adverse events (hyperglycemia and
hypertension) and complications secondary to MAS such as
pneumothorax.
After removing duplicates, full texts
of potential eligible articles, identified from their abstracts,
were obtained and assessed for inclusion.
Data extraction: Two authors (TY, BJ)
independently extracted the data using a pre-designed data
extraction form. Differences were resolved by consensus or by
involving the third author (PS).
Quality Assessment: Assessment was done
independently by TY and BJ, using Cochrane collaboration risk of
bias (ROB) assessment tool for RCTs [20], which is based on the
domains: random number generation, allocation concealment,
blinding of intervention and outcome assessors, completeness of
follow up, selectivity of reporting and other potential biases.
Accordingly, ROB was assigned as low, unclear and high risk.
[20].
Statistical analyses: The
meta-analysis was performed using RevMan 5 software. Forest
plots were calculated using weighted scores and a random effects
model (REM, Mantel Haenszel method). We employed REM to account
for heterogeneity across studies. Between-studies heterogeneity
was assessed with a chi-square test and the I 2
statistic. A P-value of < 0.1 for the chi-square
statistic indicated significant heterogeneity. For the I2
statistic, values <25% were considered low heterogeneity, 25-50%
moderate heterogeneity, >75% high heterogeneity [20,22]. For
studies that presented data as median and interquartile range,
we estimated the mean and standard deviation using the minimum
and maximum values as well as the interquartile ranges [24]. To
combine means and standard deviations, we used calculations
provided by the Cochrane handbook [20]. Effect size was reported
as relative risk (RR) and associated 95% confidence interval
(CI) or mean difference (MD) and 95% CI as appropriate.
Subgroup analysis comparing different modes
of administration of steroids: systemic (intravenous) and
inhaled steroids vs placebo or no intervention was also
performed.
Key information about the study including
quality of evidence, details of the intervention and summary of
outcome data were included in the summary of findings table
according to the Grading of Recommendations, Assessment,
Development and Evaluation (GRADE) guidelines. Grading
of evidence was performed with the online tool GradePro GDT
[23].
RESULTS
A total of nine RCTs, involving 758 newborns
were included in this systematic review and meta-analysis (Fig.1).
The characteristics of the included studies are summarized in
Table I. Seven RCTs assessed the effects of systemic
steroids [9-10,13-14,17-19] while four studies investigated
inhaled steroids for MAS [13-16]. Two studies conducted a three
arm RCT comparing systemic steroids, inhaled steroids and
placebo [13,14]. Among the studies assessing systemic steroids,
one used intravenous (IV) hydrocortisone [9], three compared IV
methylprednisolone [13-14,19] and three examined IV
dexamethasone, in comparison to no intervention or placebo
[10,17-18]. Four studies compared the role of inhaled budesonide
vs placebo or nebulized saline [13-16].
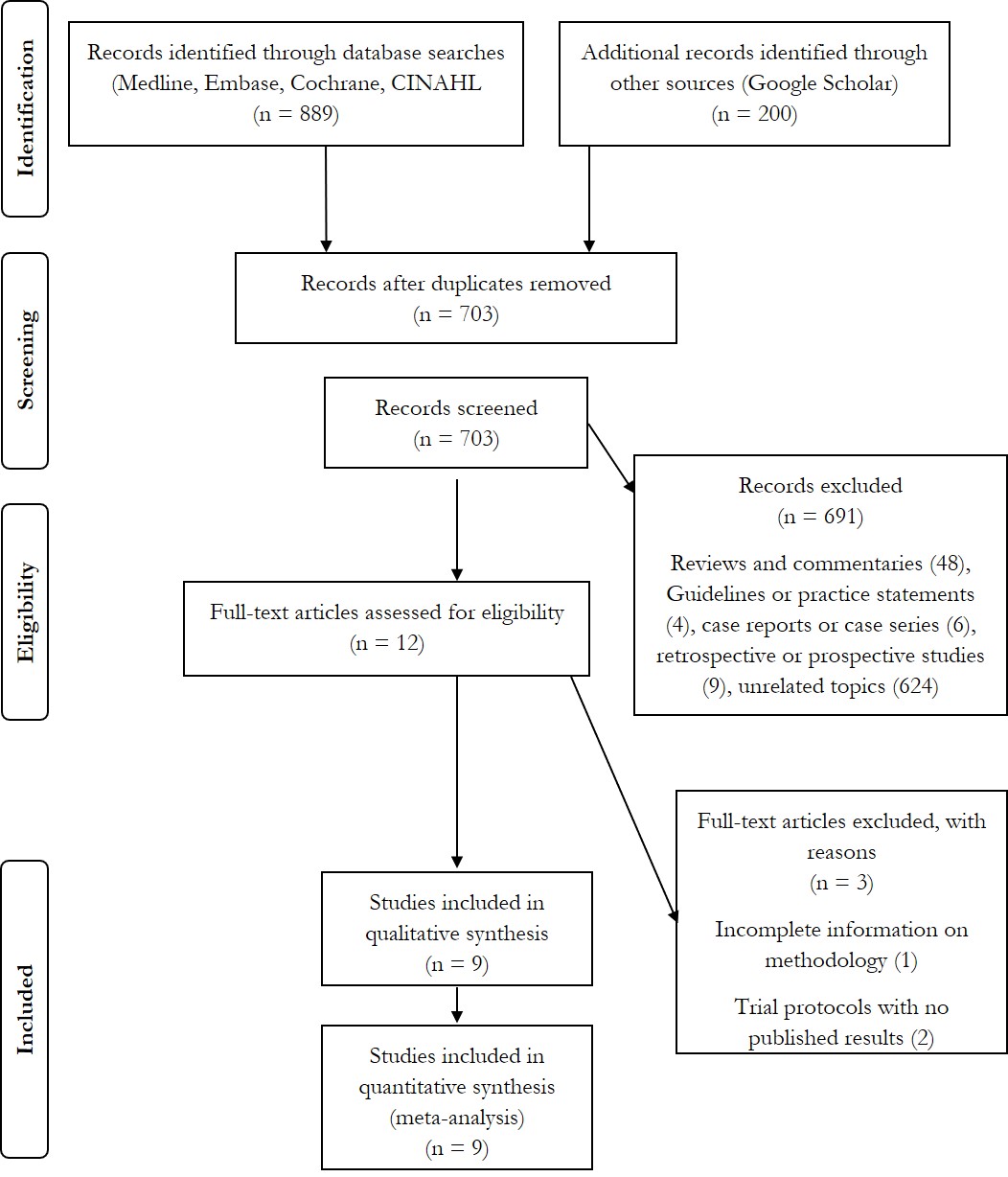 |
|
Fig.1 Flow diagram of search
strategy and study selection.
|
Using the Cochrane ROB tool, we found that
(7/9) 78% of studies had unclear ROB for allocation concealment
and (3/9) 33% had unclear ROB for random sequence generation. In
the domain of blinding of participants, (5/9) 56% of studies had
unclear risk and one study had high risk. For blinding of
outcome assessors, 78% of studies had unclear ROB. Detailed
quality assessment of the studies is shown in
Supp. Table
II.
Meta-analysis of 7 RCTs (n=423) (Supp.
Fig. 1) showed no differences in mortality among
newborns with MAS treated with steroids compared to the control
group [RR (95% CI) 0.59; (0.28, 1.23); P=0.16]
[9,10,13-16,18]. The GRADE of evidence was low due to risk of
bias and imprecision.
Analysis of duration of hospitalization,
reported in 7 studies (642 participants) [10,13-17,19] showed no
statistically significant difference between the steroid-treated
and control group [MD (95% CI) –2.58 (–5.25,0.08) days; P=0.06]
(Table II). The duration of oxygen support was also not
different between the groups [MD (95% CI) –1.38 (–3.23 to 0.48)
days; P=0.15] (Table II) [9-10,13-15,18]. Though
pneumothorax episodes were decreased, it was not significantly
different in the inhaled steroid group compared to control [RR
(95% CI) 0.29 (0.06 to 1.38); P=0.12] (Table II)
[13,15,16]. Regarding mechanical ventilation, while one study
assessed the duration of mechanical ventilation [10], the other
assessed the need for it [17], thereby making meta-analysis
difficult.
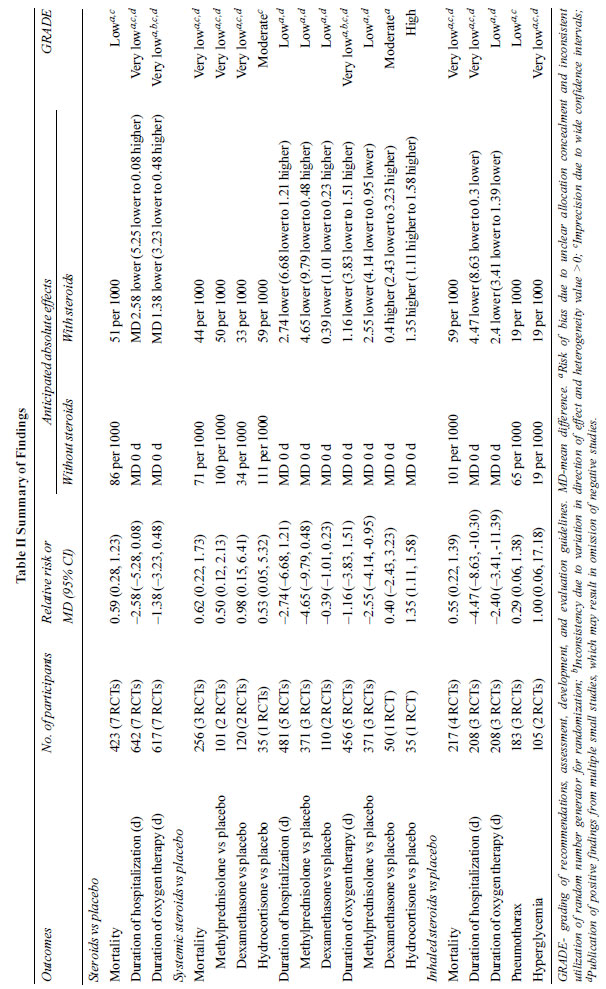 |
The rates of side effects with steroids were
not consistently reported. Two trials showed no difference in
hyperglycemia between the control and treatment groups during
the intervention [RR (95% CI) 1.00 (0.06 to 17.18); P=1.00] (Table
II) [13,19], and one trial reported no events of
hypertension [13].
Subgroup analysis: In subgroup analysis,
inhaled budesonide reduced the duration of hospital stay as well
as mean duration of oxygen support [13-16] (Supp. Fig. 2,3)
Similarly, methylprednisolone treated infants showed a
significant decrease in duration of oxygen support compared to
placebo or no intervention [13-14,18] (Supp. Fig. 4).
Quality of evidence: Using GRADE
assessment, the overall quality of evidence for all outcomes was
very low to low, due to the high risk of bias, especially
selection bias, as allocation concealment and random sequence
generation were not reported in the studies. Inconsistency was
present as trials used different types of systemic steroids,
showing different results. No indirectness was detected.
Imprecision was present due to wide confidence intervals.
Publication bias was not assessed as we had only 7 RCTs for the
primary outcome in this review.
DISCUSSION
In this updated systematic review of 9 RCTs
[9,10,13-19], we found that overall, steroids did not
significantly decrease mortality in infants with MAS compared to
controls. However, inhaled budesonide was found to decrease the
duration of hospitalization, while both inhaled budesonide and
IV methylprednisolone significantly decreased the duration of
oxygen therapy for infants with MAS. Quality of evidence was
very low to low due to the small number of trials, high risk of
bias and heterogeneity in study interventions.
Animal models of MAS have shown that steroids
administered locally or systemically resulted in decreased
histologic evidence of pulmonary inflammation and improved
oxygenation [11]. Intratracheal steroids decreased neutrophil
migration, reduced reactive oxidative damage and subsequently
decreased pulmonary tissue necrosis in piglets and rabbits with
meconium induced lung injury [11,12]. Thus, there is a biologic
plausibility regarding the effect of steroids in neonates with
MAS. Even in this review, we identified some positive effects of
inhaled budesonide and methylprednisolone in terms of duration
of hospital stay and duration of oxygen therapy. Further,
inhaled budesonide has the added advantage of avoiding the
complications of systemic steroids such as hyperglycemia and
hypertension as well as the requirement for IV access, the
possibility of infiltration injuries or the risk of IV
associated infections. In contrast, Yeh, et al. [9], reported
that hydrocortisone increased the duration of oxygen support,
which could be explained by differing potency of different
steroid compounds. Moreover, methylprednisolone and inhaled
budesonide were administered for about seven days, while in the
study by Yeh, et al. [9], hydrocortisone was administered for
two days. The severity of MAS was an important confounding
factor, which may explain the observed differences in the
effects of the steroid treatments.
Long-term effects of steroids like
neurodevelop-mental outcomes, could not be assessed in this
review due to lack of information. Though two of the studies
reported follow-up of patients at 3 or 6 months after therapy
[13,19], the method for assessing neurodevelopment was not
described in one study [13] and the other described reduction in
the composite longterm outcome of bronchopulmonary dysplasia and
cerebral palsy [19] without mentioning individual complications.
The limitation of this review is that the
included RCTs are small studies with very low to low quality of
evidence, due to high risk of bias in different domains. We
identified inconsistent reporting of additional outcomes such as
duration of non-invasive ventilation, length of mechanical
ventilation, use of iNO or need for ECMO, which could be due to
the studies being conducted in low- or middle-income countries
with limited access to iNO or ECMO. Another limitation is that
the degree of severity of MAS varied substantially across
studies with mortality ranging between 0-15.7%. The studies did
not report the effect of steroids with respect to severity of
MAS. Thus, the generalizability of this study to the full
spectrum of severity of MAS is limited.
In neonates with meconium aspiration
syndrome, low quality evidence suggests that steroid therapy
does not reduce mortality. Very low-quality evidence suggests
that inhaled budesonide reduces hospital stay while both
methylprednisolone and inhaled budesonide reduce the duration of
oxygen support. However, number of trials assessing these
interventions was small. Further large, multicenter randomized
controlled trials assessing the efficacy as well as short- and
long-term outcomes of steroids for MAS are needed.
Acknowledgements: Dr Estelle Gauda
(Division of Neonatology, Hospital for Sick Children) for her
assistance in conceiving this research question and insights
into the research topic. Chris Walsh (Sinai Health Systems,
Library Services) for his assistance in conducting the
literature search.
Funding: None; Competing interests:
None stated.
REFERENCES
1. Fanaroff AA. Meconium aspiration syndrome:
Historical aspects. J Perinatol. 2008;28:S3.
2. Edwards EM, Lakshminrushimha S, Ehret DE,
Horbar JD. NICU admissions for meconium aspiration syndrome
before and after neonatal resuscitation program suctioning
guideline change. Children 2019;6:1-8.
3. Sivanandan S, Agarwal R, Sethi A.
Respiratory distress in term neonates in low-resource settings.
Sem Fet Neonat Med. 2017;22:260-66.
4. Thorton PD, Campbell RT, Mogos MF, Klima
CS, Parsson J, Strid M. Meconium aspiration syndrome: incidence
and outcomes using discharge data. Early Hum Dat.
2018;136:21-26.
5. Chettri S, Bhat BV, Adhisivam B. Current
concepts in the management of meconium aspiration syndrome.
Indian J Pediatr 2016;83:1125-30.
6. Kelly LE, Shivananda S, Murthy P,
Srinivasjois R, Shah PS. Antibiotics for neonates born through
meconium-stained amniotic fluid. Cochrane Database of Systematic
Reviews 2017;6:CD006183.
7. Natarajan CK, Sankar MJ, Jain K, Agarwal
R, Paul VK. Surfactant therapy and antibiotics in neonates with
meconium aspiration syndrome: A systematic review and
meta-analysis. J Perinatol 2016l36:S49-S54.
8. Ward MC, Sinn JKH. Steroid therapy for
meconium aspiration in newborn infants. Cochrane Database of
Systematic Reviews. 2003;4:CD003485.
9. Yeh TF, Srinivasan G, Harris V, Pildes RS.
Hydrocortisone therapy in meconium aspiration syndrome: a
controlled study. J Pediatr. 1977;90:140-43.
10. Wu JM, Yeh TF, Wang JY, et al. The Role
of pulmonary inflammation in the development of pulmonary
hypertension in newborn with meconium aspiration syndrome.
Pediatri Pulm Suppl. 1999;18:205-08.
11. Lin C, Jeng M, Yang Y, Hsiao Y, Kou YR.
Comparison of different dosing strategies of intratracheally
instilled budesonide on meconium injured piglet lungs. Pediatr
Pulmonol 2017;52:891-899.
12. Mokra D, Mokry J, Drgova A, Petraskova M,
Bulikova J, Calkovska A. Intratracheally administered
corticosteroids improve lung function in meconium-instilled
rabbits. J Physiol Pharmacol. 2007;58:389-98.
13. Basu S, Kumar A, Bhatia BD, Satya K,
Singh TB. Role of steroids on the clinical course and outcome of
meconium aspiration syndrome – A randomized controlled trial. J
Trop Pediatr. 2007;53:331-37.
14. Tripathi S, Saili A. The effect of
steroids on the clinical course and outcome of neonates with
meconium aspiration syndrome. J Trop Pediatr. 2007;53:8-12.
15. Garg N, Choudhary M, Sharma D, Dabi D,
Choudhary JS, Choudhary SK. The role of early inhaled budesonide
therapy in meconium aspiration in term newborns: A randomized
control study. J Matern Fetal Neonatal Med. 2016;29:36-40.
16. Suresh R, Sudha R, Pinto N, Pradeep N.
Effect of nebulized budesonide in improving the clinical outcome
of neonates with meconium aspiration syndrome. Int J Biol Med
Res. 2015;6:4941-45.
17. Sangeetha T, Ramanathan R, Yogavalli S.
Effectiveness of steroid therapy in newborns with meconium
aspiration syndrome. J Med Sci Clin Res. 2017;5:22587-90.
18. Patil MM, Lakhkar BB, Patil SV.
Dexamethasone and outcome of meconium aspiration syndrome:
Vijayapur and Karnataka experience. Sri Lanka J Child Health.
2018;47:21-26.
19. Rana KS, Konar MC, Islam K, Barik KL,
Nayek K, Datta AK. Study on effects of steroids on clinical
course, short-term and long-term outcomes in neonates with
meconium aspiration syndrome. J Neonat Nurs. 2018;24:257-60.
20. Higgins JPT, Green S (editors). Cochrane
Handbook for Systematic Reviews of Interventions Version 5.1.0
[updated March 2011]. The Cochrane Collaboration, 2011. Accessed
April 14, 2020. Available from www.handbook.cochrane.org
21. Liberati A, Altman DG, Tetzlaff J, et al.
The PRISMA statement for reporting systematic reviews and
meta-analyses of studies that evaluate healthcare interventions:
explanations and elaboration. BMJ. 2009;339:b2700.
22. Higgins JPT, Thompson SG, Deeks JJ,
Altman DG. Measuring inconsistency in meta-analysis. BMJ
2003;327:557-560.
23. Schunemann H, Brozek J, Guyatt G, Oxman A
(editors). Guideline Development Tool [updated October 2013].
GRADE working group. Accessed April 23, 2020. Available from
www.gradepro.org
24. Wan X, Wang W, Liu J, Tong T. Estimating
the sample mean and standard deviation form the sample size,
median, range and/or interquartile range. BMC Med Res Method.
2014;14:135.
25. Estrany XC. European Clinical Trials
Register [Internet]: Amsterdam (Netherlands). Collegi Oficial de
Metges de Barcelona (Spain): 2006 – Identifier: 2005-002687-29.
Single-dose dexamethasone and/or bronchoalveolar lavage with
diluted surfactant in the treatment of severe meconium
aspiration syndrome. Accessed May 9, 2020. Available from
https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-002687-29/ES
26. Rajesh Varma CP. Clinical Trials Register
India [Internet]. Swami Dayanand Hospital (India): 2019 –
Identifier CTRI/2019/06/019947 Role of budesonide nebulisation
in neonates with meconium aspiration syndrome. Accessed May 23,
2020. Available from http://www.ctri.nic.in/Clinicaltrials/
pmaindet2.php?trialid=32827
27. Davey AM, Kueser TJ, Turner HF.
Randomized controlled trial of early dexamethasone therapy in
the treatment of meconium aspiration syndrome. Pediatr Res.
1995;37:329.
|
|
|
 |
|

