|
|
|
Indian Pediatr 2021;58:358-362 |
 |
Outcome of Children
Admitted With SARS-CoV-2 Infection: Experiences From a Pediatric
Public Hospital
|
|
Sudha Rao, Vrushabh Gavali, Shakuntala S Prabhu, Radhika Mathur,
Larissa Robert Dabre,
Sanjay B Prabhu and Minnie Bodhanwala
From Department of Pediatrics, Bai Jerbai Wadia Hospital for
Children, Mumbai, Maharashtra, India.
Correspondence to: Dr. Sudha Rao, Professor and, Head Department of
Pediatrics, Bai Jerbai Wadia Hospital for Children, Acharya Dhonde Marg,
Parel, Mumbai, India.
Email: [email protected]
Received: September 17, 2020;
Initial review: October 5, 2020;
Accepted: January 10, 2021.
Published online: January 11, 2021;
PII: S097475591600280
|
Objective :
To
study clinical characteristics and outcome of children with admitted to
a paediatric hospital in Mumbai, India. Method: Review of
medical records of 969 children admitted between 19 March and 7 August,
2020, to assess the clinico-demographic characteristics, disease
severity and factors predicting outcome in COVID-19 children. Variables
were compared between children who were previously healthy (Group I) and
those with co-morbidity (Group II). Results: 123 (71 boys)
children with median (IQR) age of 3 (0.7– 6) years were admitted, of
which 47 (38%) had co-morbidities. 39 (32 %) children required intensive
care and 14 (11.4%) died. Male sex, respiratory manifestation, oxygen
saturation <94% at admission, mechanical ventilation, inotrope, hospital
stay of <10 days were independent predictors of mortality. Oxygen
saturation <94% at admission (OR 35.9, 95% CI 1.5-856) and hospital stay
<10 days (OR 9.1, 95% CI 1.04-99.1) were significant. Conclusion:
COVID-19 in children with co-morbidities causes severe disease.
Association of mortality with oxygen saturation by pulse oximeter <94%
on admission, and hospital stay <10 days, needs further evaluation.
Keywords: Co-morbidities, Mortality, Multisystem inflammatory
syndrome in children (MIS-C), Prognosis.
|
|
Severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) infection, leading to COVID-19
disease pandemic, has spread all over India and the world.
Mumbai Metropolitan Region (MMR) and the City of Mumbai is the
worst affected hotspot in India. Our center, a tertiary care,
public, specialist hospital, received many children with
SARS-CoV-2 from March 19, 2020. Many vulnerable children with
co-morbid conditions like heart disease, malnutrition,
malignancy, diabetes, chronic kidney disorder, etc. also
presented for acute inter current emergencies. This
retrospective study presents the demographic, clinical
characteristics, treatment and, outcome, care of neonates and
children with SARS-CoV-2 positivity from our center.
METHOD
Retrospective medical record review of all
children admitted to the hospital between March 19, 2020 and
August 7, 2020 was done. Approval of institutional ethics
committee obtained. All children with reverse
transcriptase-polymerase chain reaction (RT-PCR) positive for
SARS-CoV-2 were studied.
As per institutional protocol, derived from
national guidelines [1], every child requiring admission was
tested by RT-PCR for SARS-CoV-2 from an Indian Council of
Medical Research (ICMR) recognized laboratory. Children who
tested positive were admitted to the isolation ward, specially
created as per national guidelines [2]. Historical details and
pre-existing co-morbidities were recorded. COVID-19 disease
characterization was done as per guidelines [1]. Multisystem
inflammatory syndrome in children (MIS-C) and Kawasaki disease
(KD) were defined as per standard definition [3,4].
Institutional protocol of care created based on ICMR /GOI
recommendations was followed. Some cases of MIS-C/KD included in
this study have been previously published [5].
Laboratory investigations and imaging studies
were carried as necessary. Therapeutic principles included
general supportive therapy, active control of fever, respiratory
support with oxygen and/or ventilation as necessary, vasoactive
drugs in shock, and active monitoring of organ system
dysfunctions. Remdesivir was given to children above 12 years of
age with COVID-19 pneumonia. Younger children received it on
compassionate grounds with risk explained and an informed
consent taken. Intravenous immunoglobulin, pulse methyl-prednisolone,
and anticoagulation with low-molecular-weight heparin were used
as per protocol. Repeat testing for SARS-CoV-2 PCR and discharge
criteria were followed as per guidelines [1]. Time taken to PCR
negativity and duration of hospital stay was noted. Treatment
outcomes were defined as discharged or died.
SARS-CoV-2 positive children in this cohort
were classified into Group I comprising of previously healthy
children, and Group II with children having co-morbidities like
heart disease, diabetes, malignancy, malnutrition, renal,
hepatobiliary, neurological, surgical/orthopedic conditions,
etc. Variables were compared between the groups.
Statistical analyses: Data was entered in
MS Excel, and coded and analyzed in statistical software STATA,
version 10.1 (Stata Corp.). Pearson Chi-square test was used for
assessing significance of association between outcome
(mortality/discharge) and exposure variables/predictors.
Binomial test for difference in proportions was also used to
compare proportions in sub-groups or categories in two groups.
Student t-test or Mann–Whitney test was performed to assess
significance of difference in means or medians in two
independent groups. Binary multiple logistic regression model
was applied to identify predictors of mortality accounting for
the role of other factors, wherein adjusted odds ratio (OR) and
95% Confidence Intervals (CI) were estimated. A P value
of <0.05 was considered statistically significant for all the
comparisons.
RESULTS
Of 969 children admitted during the study
period, 123 (12.8%) tested positive for SARS-CoV-2 including 16
(13%) extramural neonates. Five (4.1%) had a history of travel.
The median (IQR) age at presentation was 3 (0.7–6.0) year with a
male: female ratio of 1.36 (Table I).
Table I Baseline Characteristics, Clinical Profile and Outcome in Children With SARS-CoV-2 Infection (N=123)
|
All children |
Previously |
With co- |
|
|
healthy |
morbidity |
|
|
(n=76) |
(n=47) |
| Male |
71 (57.7) |
43 (56.6) |
28 (59.6) |
| Age wise distribution |
|
|
|
| < 1mo of age |
16 (13.0) |
12 (15.8) |
4 (8.5) |
| 1mo-1 y |
31 (25.2) |
20 (26.3) |
11 (23.4) |
| 1y-5y |
39 (31.7) |
25 (32.9) |
14 (29.8) |
| 5-10 y |
26 (21.1) |
17 (22.4) |
9 (19.2) |
| >10yc |
11 (8.9) |
2 (2.6) |
9 (19.2) |
| Symptoms at presentation |
|
|
|
| Asymptomaticc |
27 (21.9) |
8 (13.2) |
19 (36.2) |
| Fever |
24 (19.5) |
16 (21.0) |
8 (17.0) |
| Upper respiratory |
5 (4.1) |
3 (3.9) |
2 (4.3) |
| Lower respiratory |
25 (20.3) |
18 (23.7) |
7 (14.9) |
| Gastrointestinal |
15 (12.2) |
12 (15.8) |
3 (6.4) |
| Seizuresb |
13 (10.6) |
12 (15.8) |
1 (2.1) |
| Othersb |
14 (11.4) |
5 (6.6) |
9 (19.2) |
| Radiology |
n=114 |
n=73 |
n=41 |
| Abnormal X-ray chest |
23 (20) |
16 (22.0) |
7 (27.7) |
| Disease severity |
|
|
|
| Mildd |
54 (43.9) |
50 (65.8) |
4 (8.5) |
| Moderated |
26 (21.1) |
4 (5.3) |
22 (46.8) |
| Severeb |
32 (26.0) |
14 (18.4) |
18 (38.3) |
|
MIS-C/KDc |
11 (8.9) |
8 (10.5) |
3 (6.4) |
| Need for intensive care |
39 (31.7) |
24 (31.6) |
15 (31.9) |
| Respiratory support |
|
|
|
| Only oxygen |
20 (16.3) |
13 (17.1) |
7 (14.9) |
| Non-invasive ventilation |
6 (4.9) |
5 (6.7) |
1 (2.1) |
| Invasive ventilation |
13 (10.6) |
8 (10.5) |
5 (10.6) |
| Vasoactive drugs used |
17 (13.8) |
11 (14.5) |
6 (12.8) |
| Outcome |
|
|
|
| Death |
14 (11.4) |
6 (7.9) |
8 (17.0) |
| Discharge |
105(85.4) |
68 (89.5) |
37 (78.7) |
| Still admitted |
4 (4) |
2 (2.6) |
2(4.25) |
| Values in no. (%)
except amedian (IQR). For comparison between groups bP<0.05,
cP<0.01, dP<0.001. MIS-C/KD-Multisystem Inflammatory
Syndrome in Children/ Kawasaki disease. |
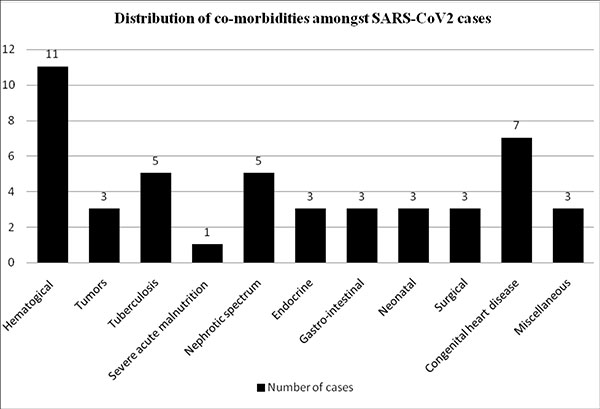 |
|
Fig. 1 Distribution of
co-morbidities amongst children with SARS-CoV-2
infection.
|
Seventy six (62%) children belonged to Group
I and 47 (38%) Group II. Distribution of underlying illness in
Group II is shown in Fig. 1. Children with severe
COVID-19 had underlying hemato-oncological, renal and cardiac
disorders. Children in Group I presented at a younger age than
those in Group II [median (IQR) 1.7 (10.5–5.25) vs. 4 (0.8-9)
years; P=0.052]; 27 (22%) children were asymptomatic.
Fever in 24 (20%) or respiratory symptoms in 30 (24%) children
were common presenting symptoms, and seizures in 13 (10%) and
gastrointestinal symptoms in 15 (12.2%) children were the
atypical presentations. Six (5%) children presented with
injuries like head injury /limb fracture. Interestingly, severe
COVID-19 disease was seen more in Group II whereas MIS-C/KD was
more in Group I (Table I).
On admission, mean (SD) pulse oximeter
saturation (SpO2) and the blood neutrophil: lymphocyte ratio was
lower, respectively in Group I than Group II [94.7 (7.1) vs 96.5
(6.1); P=0.09] and [2.6 (3.6) vs 4.5 (5.1); P=0.09].
Chest radiograph was done in 114 (93%) cases, it was abnormal in
23 (20%) with bilateral haziness, consolidation and pleural
effusion being the common abnormalities.
Eighty four (68.3%) children did not require
respiratory support. More number of children in Group I (n=13/19)
required ventilator care. Vasoactive drugs required in 17 (14%)
cases and 11were from Group I (Table I). Severe COVID-19
pneumonia (n=10/39), circulatory collapse (n=5/39),
MIS-C/KD (n=8/39), worsening of underlying disease (n=16/39)
as indications, 39 (32%) children needed intensive care, which
was similar in Groups I and II. Left ventricular dysfunction (n=6),
dilatation of coronaries (n=2) were the echocardiography
findings in eight children with MIS-C/KD; 4 (50%) children
received IVIG within 48 hours. Remdesivir was given to two
children with severe COVID-19 pneumonia.
While compiling the study, 4 children were
still admitted. The median duration of PCR negativity was 5 days
(range, 3-15 days). 105 (88%) patients were discharged. The
median duration of PCR negativity was 5 days (range, 3-15 days);
105 (88%) patients were discharged. The median (range) length of
hospital stay was 9 days (4-17 days), which did not differ
significantly between Groups I and II (Table I).
There were 14 (11.4%) deaths of which 3
(21.5%) were neonates. Four children in Group II who died had
underlying malignancy. Male sex, SpO2<94% at admission, abnormal
chest X-ray, need for respiratory support, need for
vasoactive support, need for intensive care and the duration of
hospital stay were predictors of mortality on univariate
analysis (Table II). SpO2<94% at admission [OR (95% CI)
9.1 (1.04–99.1); P=0.04] and hospital stay of less than 9
days [OR (95% CI) 35.9 (1.5-856.0); P=0.02] were
predictors of mortality on regression analysis.
Table II Predictors of Outcome in Pediatric Inpatients With SARS-CoV-2 Infection (N=119)
| Factors |
Death |
Discharge |
OR |
|
(n=14) |
(n=105) |
95% CI |
| Age at presentation
≥3 y |
7 (50) |
56 (88.9) |
0.88 |
|
|
|
(0.24- 3.15) |
|
Male sexa |
11 (78.6) |
58 (55.2) |
4.52 |
|
|
|
(1.1-26.4) |
| Asymptomatic |
0 |
27 (25.7) |
- |
| Respiratory symptoms a |
10 (71.4) |
41 (39.1) |
- |
|
Normal X-ray chest,b |
5 (35.7) |
82 (80) |
7.2 |
| n=110 |
|
|
(1.9-29.7) |
|
SpO2 <94% at admissionb |
8 (57.1) |
15 (14.3) |
8.0 |
|
|
|
(2.0-31.6) |
|
Respiratory supportb |
12 (85.7) |
25 (23.8) |
19.2 |
|
|
|
(3.8- 182.5) |
|
Use of vasoactive drugs
|
7 (50.0) |
10 (9.5) |
19.5 |
|
|
|
(2.3- 38.5) |
|
Need for intensive careb |
12 (85.7) |
26 (24.8) |
18.2 |
|
|
|
(3.6- 173.2) |
| Hospital stay
≥9 d |
7 (87.5) |
43 (50.0) |
7 |
|
|
|
(0.8- 322.6) |
| All values in no. (%);
aP<0.05, bP=0.001. |
DISCUSSION
The study highlights the clinical
characteristics, disease progression, and outcome of 123
children admitted with COVID-19. As admitted children were
enrolled, the data likely represents individuals from the
moderate-to-severe end of the disease spectrum.
The proportion of previously healthy children
was 62%. In a study from Columbia Pediatric COVID-19 management
group co-morbidities defined as obesity, asthma, infancy or
immune suppression were studied [6].Recent data from US studied
chronic lung disease, cardiovascular disease and immune
suppression as the common co-morbidities [7].Twenty seven
(21.7%) children were asymptomatic comparable to the
meta-analysis where 23% were asymptomatic [8]. Initial studies
from China reported 4.1-50% cases to be asymptomatic, while 58%
were asymptomatic in a study from Pune [9,10].Fever and
respiratory symptoms were the common presenting symptoms as also
found by others [8-10]. Atypical presentations like seizures
(10.6%), gastrointestinal symptoms (12.2%) were more common in
this series as compared to other studies[7,10,11]. More children
in our cohort had severe disease as compared to only 1% as
reported in recent retrospective study from China[12].Children
with underlying hemato-oncological, renal or cardiac disorders
had severe disease. Interestingly, the immune response of
COVID-19, the MIS-C/KD was found more in Group I than Group II.
This has been reported in other studies also[5,13].Presence of
comorbidity dysregulates or blunts the immunological host
responses causing severe infection but a hyper-inflammatory
immune response like MIS-C/KD is not seen.
Need for intensive care in our series is
similar to that reported in literature [14]. Adult studies
suggest presence of co-morbidities as an important predictor of
need for intensive care[15], which was not found by us. Children
requiring mechanical ventilation (15.5%) were fewer than the
cohort from USA [6,16] as we had more non-respiratory
presentations.
A study of children from the European cohort
concluded that neonates, male sex, pre-existing medical
conditions, fever, lower respiratory tract infection,
radiological changes of pneumonia or ARDS, and viral
co-infection were associated with more severe course on
univariate analysis; however, these were not correlated to
mortality [11]. In our cohort, male sex, hypoxia (SpO2 <94%) on
admission, need for respiratory support, inotropes, intensive
care, length of hospital stay <10 days was significantly
associated with mortality. Male gender has been associated with
a higher risk of severe disease and mortality because of higher
ACE-2 receptor expression [17]. On regression analysis, SpO2
<94% on admission and length of hospital stay of <10 days were
predictors of mortality and not the presence of co-morbidities.
This need to be corroborated with a bigger sample size.
Experience from adult studies has shown mortality within 1 to 2
weeks of ICU admission [15].
As a retrospective study, certain important
parameters like onset of symptoms from day of contact, source of
infection, and exact duration of COVID-19 RT-PCR positivity in
all children could not be assessed.
To conclude, pediatric COVID-19 although
considered a mild illness, children with co morbidity manifest
with severe disease. Male sex, hypoxia on admission, need for
intensive care, ventilator support, inotrope, hospital stay of
<10 days are predictors of mortality. Policy of testing all
admitted children for COVID-19 helps identify and segregate the
cases, provide protocol based care, characterize the severity,
initiate prompt treatment and improve outcome.
Contributors: SR,SSP,SBP: conceived,
designed the study, finalised the manuscript; SR,VG,RM,
LRD,SBP,SSP,MB: data collection, data analysis;
SR,VG,RM,LRD,SSP,SBP: Literature search, interpretation of data,
writing manuscript. All authors approved the final manuscript.
Ethics clearance: Institutional Ethics
Committee Bai Jerbai Wadia Hospital for Children; No. IEC-BJWHC/
89/2020, dated 26 August, 2020.
Funding: None; Competing interests:
None stated.
| |
|
WHAT IS ALREADY KNOWN?
• Neonates, male gender, pre-existing
medical conditions, fever, lower respiratory tract
infection, radiological changes suggestive of pneumonia
or ARDS, and viral co-infection were associated with
more severe course.
WHAT THIS STUDY ADDS?
• Children with underlying medical
illnesses have significantly severe COVID-19 disease.
• Male gender, hypoxia (SpO2 <94%)
on admission, need for respiratory support, need for
vasoactive drugs, ICU care, and length of hospital stay
of <10 days is significantly associated with mortality.
|
REFERENCES
1. Directorate General of Health Services.
Revised National Clinical Management Guideline for COVID-19. New
Delhi (IN): Ministry of Health & Family Welfare. Available at
www.mohfw.gov.in.
2. National Centre for Disease Control. COVID
-19 Outbreak Guidelines for Setting up. New Delhi (IN): Ministry
of Health & Family Welfare. Available at www.mohfw.gov.in.
3. Jiang L, Tang K, Levin M, et al. COVID-19
and multisystem inflammatory syndrome in children and
adolescents. Lancet Infect Dis. 2020;20:e276-88.
4. Singh S, Jindal AK, Pilania RK. Diagnosis
of Kawasaki disease. Int J Rheum Dis. 2018;21:36-44.
5. Shobhavat L, Solomon R, Rao S, et al.
Multisystem inflammatory syndrome in children: Clinical features
and management—Intensive care experience from a pediatric public
hospital in Western India. Indian J Crit Care Med. 2020; 24:
1089–94.
6. Zachariah P, Johnson CL, Halabi KC, et al.
Epidemiology, clinical features, and disease severity in
patients with coronavirus disease 2019 (COVID-19) in a
children’s hospital in New York City, New York. JAMA Pediatr.
2020;174:e202430.
7. De Luca CD, Esposito E, Cristiani L, et
al. Covid-19 in children: A brief overview after three months
experience. Paediatr Respir Rev. 2020; 35:9-14.
8. Meena J, Yadav J, Saini L, Yadav A, Kumar
J. Clinical features and outcome of sars-cov-2 infection in
children: A systematic review and meta-analysis. Indian Pediatr.
2020;57: 820-26.
9. Dong Y, Mo X, Hu Y, et al. Epidemiology of
COVID-19 among children in China. Pediatrics. 2020
;145:e20200702
10. Sarangi B, Reddy VS, Oswal JS, et al.
Epidemiological and clinical characteristics of COVID-19 in
Indian children in the initial phase of the pandemic: A
cross-sectional study. Indian Pediatr. 2020; 57:914-17.
11. Götzinger F, Santiago-García B,
Noguera-Julián A, et al. COVID-19 in children and adolescents in
Europe: A multinational, multicentre cohort study. Lancet Child
Adolesc Health. 2020;4:653-61.
12. Guo CX, He L, Yin JY, et al.
Epidemiological and clinical features of pediatric COVID-19. BMC
Med. 2020;18:250.
13. Yonker LM, Neilan AM, Bartsch Y, et al.
Pediatric severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2): Clinical presentation, infectivity, and immune
responses. J Pediatr. 2020;227:45-52.
14. Kim L, Whitaker M, O’Halloran A, et al.
Hospitalization rates and characteristics of children aged <18
years hospitalized with laboratory-confirmed COVID-19 -
COVID-NET, 14 States, March 1-July 25, 2020. Morb Mortal Wkly
Rep. 2020;69:1081-88.
15. Yang X, Yu Y, Xu J, et al. Clinical
course and outcomes of critically ill patients with SARS-CoV-2
pneumonia in Wuhan, China: A single-centered, retrospective,
observational study. Lancet Respir Med. 2020;8:475-81.
16. Shekerdemian LS, Mahmood NR, Wolfe KK, et
al. Characteristics and outcomes of children with coronavirus
disease 2019 (COVID-19) infection admitted to US and Canadian
pediatric intensive care units. JAMA Pediatr. 2020;174:868-73.
17. Yuki K, Fujiogi M, Koutsogiannaki S.
COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:
108427.
|
|
|
 |
|

