|
|
|
Indian Pediatr 2019;56: 287-293 |
 |
Indigenously Prepared Ready-to-use
Therapeutic Food (RUTF) in Children with Severe Acute
Malnutrition
|
|
Alka Rajendra Jadhav 1,
Prachi Karnik1,
Lavina Fernandes1,
Sneha Fernandes1,
Narendra Shah2
and Mamta Manglani1
From Department of 1Pediatrics,
Lokmanya Tilak Municipal Medical College and General Hospital, and
2Centre for Technology Alternatives for Rural Areas, Indian
Institute of Technology; Mumbai, Maharashtra, India.
Correspondence to: Dr Prachi Karnik, Assistant
Professor, Department of Pediatrics, Lokmanya Tilak Municipal Medical
College and General Hospital, Sion, Mumbai, Maharashtra 400 022, India.
Email: [email protected]
Received: March 23, 2017;
Initial review: September 26, 2017;
Accepted: January 22, 2019.
Trial Registration: CTRI/2014/04/004523 (Retrospecitive
registration)
|
|
Objective: To compare efficacy of
indigenous Ready-to-use Therapeutic Food (Medical Nutrition Therapy)
with Standard Nutrition Therapy in children with Severe acute
malnutrition.
Design: Two facility-based and
two community-based models: (i) Open prospective randomized
controlled trial comparing Indigenous Ready-to-use Therapeutic Food
(Medical Nutrition Therapy) with Standard Nutrition Therapy; (ii)
Only Indigenous Ready-to-use Therapeutic Food (Medical Nutrition
Therapy); (iii) Doorstep Child Care Centre; and (iv)
Community-based Management of Acute Malnutrition.
Setting: (i) Urban Health
Center, Dharavi, Mumbai; (ii) Two day care centers of
Non-governmental Organization SNEHA – Mumbai; (iii) Urban slums,
M East and L Ward, Mumbai
Participants: 1105 children aged
6-60 months in community or hospital inpatient/ outpatient department
diagnosed as Severe Acute Malnutrition by WHO definition.
Intervention: All subjects
received either Indigenous Ready-to-use Therapeutic Food (Medical
Nutrition Therapy) or Standard Nutrition Therapy (protein calorie rich
diet) for eight weeks and followed up for next four months.
Main outcome measures: Mean rate
of weight gain (g/kg/day), target weight, change in nutritional status.
Results: Rate of weight gain was
higher (P<0.05) at 2 weeks on indigenous Ready-to-use Therapeutic
Food (Medical Nutrition Therapy) (5.63 g/kg/day) as compared to Standard
Nutrition Therapy (3.43 g/kg/day). 61.2% subjects achieved target weight
compared to 47.7% controls. At 8 weeks, 82.8% subjects recovered from
Severe Acute Malnutrition compared to 19.3% controls (P<0.005).
The results obtained in community were comparable to facility-based
indigenous Ready-to-use Therapeutic Food (Medical Nutrition Therapy).
The morbidity was less in study group at follow-up.
Conclusions: Indigenous
Ready-to-use Therapeutic Food (Medical Nutrition Therapy) appeared to be
superior to Standard Nutrition Therapy in promoting weight gain in
children with Severe Acute Malnutrition.
Keywords: Medical Nutrition
Therapy, Micronutrients, Nutritional rehabilitation, Protein energy
malnutrition. Ready-to-use-food.
|
|
M
alnutrition is a major health
concern in
Indian children, not only in rural areas, but
also in urban slums. Every third
malnourished child in the world lives in India [1]. Globally, around 20
million children under 5 years of age have Severe acute malnutrition
(SAM) and 40 percent of these (8 million) are in India. This accounts
for 6.4% of all Indian children under five years of age.
Conventionally malnutrition was attributed to protein
and/or energy deficiency .
Newer research reveals that it is primarily due to deficiency of type II
nutrients leading to loss of appetite, growth cessation, reductive
adaptation to environmental stress, oxidative stress or infection [2].
Most of these children also have deficiency of type I nutrients that
affect specific physiologic functions.
The standard of care recommended by WHO in management
of SAM is Ready-to-use Therapeutic Foods (RUTF) containing balanced
amounts of all necessary nutrients (Type 1 and 2) in the bioavailable
form. Evidence for feasibility, acceptability, safety and efficacy of
RUTF is lacking in India. We decided to address this by devising a
locally produced RUTF termed Indigenous Ready to Use Therapeutic Food
[RUTF-I (Medical Nutrition Therapy, MNT)] [3]. This study was undertaken
to analyze the various aspects of use of RUTF-I (MNT) in facility- and
community-based management of children with SAM.
Methods
The Department of Pediatrics, LTMG Hospital
established an RUTF-I (MNT) production unit as part of its
state-of-the-art Nutrition Rehabilitation, Research and Training Centre
(NRRTC) at Urban Health Centre (UHC), Dharavi. The ingredients of RUTF-I
(MNT) were peanut butter (25%), skimmed milk powder (24%), powdered
sugar (28%), soya bean oil (21%), and micronutrients (1.6%) with
emulsifier (0.4%), which meet the WHO recommendations on RUTF
composition. 100 g of RUTF-I (MNT) provides 540 kcal and 16 g proteins
[3]. Caloric value of 100 g Standard Nutrition Therapy, SNT (comprising
of milk with sugar and oil, boiled eggs, banana, rice green gram
porridge with vegetables, jaggery and oil) was 100 kcal with 3 g
proteins. Regular batch- testing of RUTF-I (MNT) was done for Aflatoxin
assay and bacterial and fungal culture.
All children aged 6-60 months in the community
(Doorstep Childcare Center, DCC/Community Management of Acute
Malnutrition, CMAM model) or in the hospital inpatient or outpatient
department (facility-based model) diagnosed as SAM by WHO definition
(weight-for-length/height <-3 SD or mid-upper arm circumference (MUAC)
<11.5 cm and/or bilateral pitting pedal edema) were enrolled [4].
Children unable to take oral feeds or already on nutritional supplements
or with any pre-existing chronic illness were excluded. Ethics clearance
was obtained from the Institutional Ethics Committee of the institute.
The study was planned to assess four models:
First model: RUTF-I (MNT) vs SNT (April
2011 – June 2013): A prospective randomized controlled open trial was
undertaken to compare the efficacy of RUTF-I (MNT) with SNT in
hospitalized SAM children at NRRTC. NRRTC consisted of 15 bedded indoor
unit, an outdoor unit and an indigenous production unit for preparing
RUTF-I (MNT). The study was monitored by a dedicated medical officer
along with a nutritionist-cum-counseler.
We carried out an interim analysis to compare the
efficacy of RUTF-I (MNT) over SNT, which proved the superiority of
RUTF-I (MNT) over SNT. Hence, we dropped out the SNT arm of the study
and continued to give only RUTF-I (MNT) to all our SAM children as a
policy decision.
Second model: Only RUTF-I (MNT) (June 2013 – June
2015) – We continued management only with RUTF-I (MNT) and studied its
effectiveness in a facility-based model (NRRTC).
With an aim to study the feasibility of RUTF-I (MNT)
use in uncomplicated SAM children in the community, we planned Model 3
and Model 4 of the study simultaneously. We coordinated with the NGO
SNEHA (Society for Nutrition Education and Health Action) for these two
models.
Third model: DCC model (August 2012 – December
2013) – 27 DCCs were established in Dharavi, M East and L wards. DCCs
were day care centers having one trained teacher and helper each. All
eligible subjects were examined by a Medical Officer and registered for
intervention. Throughout the treatment duration, RUTF-I (MNT) was
administered under observation from Monday to Friday and RUTF-I (MNT)
for remaining two days was given at home. A community organizer (CO)
from the NGO SNEHA along with Anganwadi Sevika conducted and recorded
the monthly anthropometry. The data was then submitted to the
intervention team.
Fourth model: CMAM model (August 2013 – August
2015) – This was applied in the same geographical areas as the DCCs. One
community organizer (CO) along with one community helper was appointed
for 1000 population. The process of identification and enrolment was
similar to that of DCC. The CO visited the child daily in first week
followed by alternate days for next seven weeks. Weight was monitored
fortnightly during the treatment period and then monthly for four
months.
In the first model, 321 children were enrolled after
an informed written consent by caretakers. Detailed socio-demographic
data were obtained. After initial resuscitation and stabilization with
F75 and F100 respectively, children were subjected to the appetite test.
Those who passed the test (based on the WHO appetite test chart [5]),
were allocated into intervention and control groups to RUTF-I (MNT) or
SNT diet exclusively. The randomization was done using a computer
generated random number table by Microsoft Excel.
The intervention group received RUTF-I (MNT) at 175
kcal/kg present weight/ day for eight weeks. Caregivers received
nutritional counseling and children shifted to home diet after eight
weeks. The control group received SNT (175 kcal/kg/day). This was given
through the hospital kitchen during hospital stay and the caregiver was
trained to prepare the same at home after discharge. All children were
hospitalized for a period of two weeks or till they satisfied the WHO
discharge criteria, whichever was later.
Weight was monitored daily during hospital stay, once
a week for next six weeks, and monthly for next four months.
Height/Length and MUAC was recorded weekly for 8 weeks and then monthly
for next 4 months. The proportion of RUTF-I (MNT) consumed and morbidity
parameters (respiratory infections and diarrhea) were recorded.
Primary outcome variables were mean rate of weight
gain (gm/kg/day), proportion of children achieving target weight and
recovery from SAM status. The mean rate of weight gain (g/kg/day) was
calculated as weight gain over a defined time period divided by the
number of days. Target weight is defined by UNICEF as 15% weight gain
above the baseline weight. Recovery from SAM is defined as weight for
height more than – 3 SD or MUAC >115 mm, for the purpose of this study.
Statistical analysis: Children who
completed at least two weeks of treatment were included in the analysis.
Data from baseline, day 14, day 28, day 42, day 56, and day 180 were
used for analysis. Data were analyzed using SPSS 15.0. Distribution of
the rate of weight gain was not normally distributed. Hence non
parametric test [Friedman test for several related data (non-parametric
2 way ANOVA)] was applied to compare mean weight gain and mean rate of
weight gain at all time points. As per the requirement of the
statistical test, data with all time point values were included in the
analysis (Per protocol population). After getting significant difference
by Friedman test, Wilcoxon Signed Rank test was applied to rate of
weight gain values between two time points. All tests were two tailed.
Level of significance was taken as P=0.05.
Results
A total of 880 children who completed at least two
weeks of intervention were included in the analysis. The details of
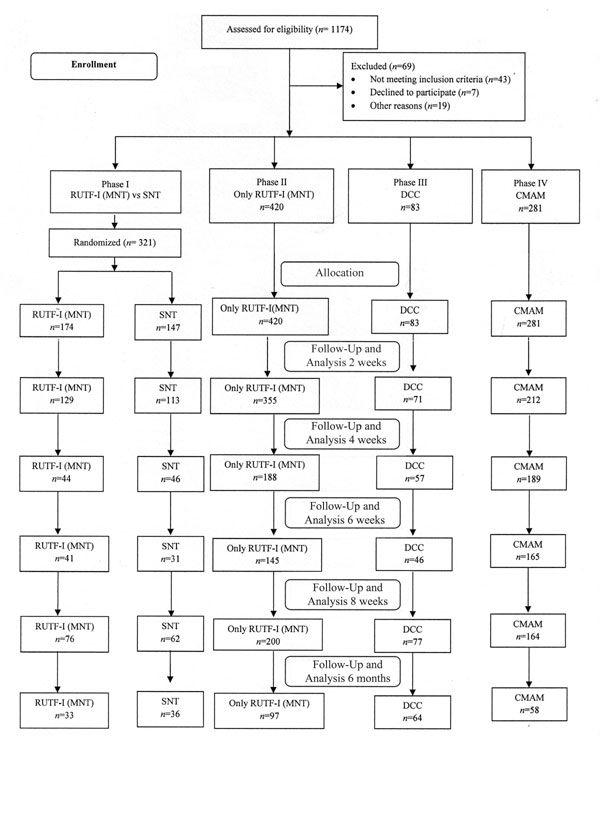
follow up at each point in time are depicted in Fig.1.
Detailed demographic data are presented in Table I. The
rise in the mean weight at every follow up during intervention was more
in all the RUTF-I (MNT) models as compared to the SNT model.
 |
|
Fig. 1 Flow of participants in
the study.
|
TABLE I Demographic and Anthropometric Parameters
|
RUTF-I (MNT) with SNT |
Only RUTF-I |
DCC (n=127) |
CMAM (n=281) |
|
RUTF-I (MNT) |
SNT (n=147) |
(MNT)(n=420) |
|
|
|
(n=174) |
|
|
|
|
|
Age |
|
|
|
|
|
|
Median (mo) |
23.28 |
29.01 |
19.83 |
20 |
26 |
|
6 mo - 1 y |
52 (29.8%) |
54 (36.7%) |
158 (37.6%) |
46 (36.2%) |
64 (22.7%) |
|
1-3 y
|
91 (52.2%) |
59 (40.1%) |
212 (50.4%) |
57 (44.8%) |
151(53.7%) |
|
3-5 y |
31 (17.8%) |
34 (23.1%) |
50 (12%) |
24 (18.8%) |
66 (23.4%) |
|
Male sex |
85 (48.8%) |
67 (45.5%) |
213 (50.7%) |
60 (47.2%) |
141 (50.1%) |
|
Anthropometry |
|
|
|
|
|
|
Weight (kg), mean (SD) |
6.7 (1.8) |
6.76 (2.9) |
6.53 |
7.25 |
7.46 |
|
Height (cm), mean (SD) |
73.6 (10.2) |
75.4 (13.4) |
71.4 |
74.2 |
77.3 |
|
MUAC (cm), mean (SD) |
11.2 (1.2) |
11.6 (1.7) |
11.4 |
- |
- |
|
RUTF-I (MNT): Indigenous Ready to use therapeutic food; SNT:
Standard nutrition therapy; DCC: Day care centre; CMAM:
Community management of acute malnutrition. |
Model 1 (RUTF-I vs SNT)
A total of 129 children on RUTF-I (MNT) and 113 on
SNT completed two weeks of treatment. The cumulative mean rate of weight
gain was 4.5 g/kg/d in RUTF-I (MNT) group and 2.9 g/kg/day in SNT group
during intervention. The mean rate of weight gain throughout the first 8
weeks was significantly higher in the RUTF-I (MNT) group compared with
the SNT group (P<0.05), and it was highest in the initial 14 days
(5.63 g/kg/day for RUTF-I (MNT) and 3.43 g/kg/day for SNT). It almost
equalized at the end of 6 months (Table II). 60.4% (78)
children in the RUTF-I (MNT) group achieved the target weight as
compared to 47.8% (54) in the SNT group. Of the 78 children who achieved
the target weight in the RUTF-I (MNT) group, 25 (32.1%) did so in the
first 2 weeks itself. In comparison, of the 54 children who achieved the
target weight in the SNT group, only 2 (3.7%) did so in the first 2
weeks (Table III). Recovery rate at the end of 8 weeks was
82.8% in RUTF-I (MNT) group. At the end of 8 weeks, only 17.1% children
on RUTF-I (MNT) (model 1) were non-responders as against 35.4% on SNT.
At the end of 6 months, only 15.1% children on RUTF-I (MNT) (model 1)
were non-responders as against 33.3% on SNT (Table IV). On
follow up, incidence of infections were 17.1% in RUTF-I (MNT) and 30.8%
in SNT (P=0.056).
TABLE II Mean Weight and Rate of Weight Gain
|
Groups |
Parameters monitored |
On admission |
2 wks |
4 wks |
6 wks |
8 wks |
6 mo |
|
Model 1 |
RUTF-I (MNT) |
Mean weight (kg) |
6.70 |
7.22 |
7.26 |
7.67 |
7.93 |
8.92 |
|
|
Rate of weight gain (g/kg/d) |
|
5.63 |
4.72 |
4.22 |
3.45 |
1.75 |
|
SNT |
Mean weight (kg) |
6.76 |
7.07 |
6.83 |
7.18 |
7.29 |
8.05 |
|
|
Rate of weight gain (g/kg/d)
|
|
3.43 |
2.82 |
2.98 |
2.38 |
1.67 |
|
P value (rate of weight gain) |
|
|
<0.05 |
<0.05 |
<0.05 |
<0.05 |
|
|
Model 2 |
Only RUTF-I (MNT) |
Mean weight (kg) |
6.54 |
6.95 |
7.24 |
7.36 |
7.65 |
8.36 |
|
|
Rate of weight gain (g/kg/d)
|
|
5.67 |
2.25 |
3.01 |
2.01 |
1.65 |
|
P value (rate of weight gain) |
|
<0.001 |
|
|
|
|
|
|
Model 3 |
DCC |
Mean weight (kg)
|
7.25 |
8.25 |
8.52 |
8.89 |
8.68 |
8.32 |
|
|
Rate of weight gain (g/kg/d)
|
|
11.14 |
4.92 |
3.77 |
0.82 |
0.06 |
|
P value (rate of weight gain) |
|
<0.001 |
0.01 |
|
|
|
|
|
Model 4 |
CMAM |
Mean weight (kg) |
7.45 |
8.42 |
8.61 |
8.69 |
8.78 |
9.38 |
|
|
Rate of weight gain (g/kg/d)
|
|
9.20 |
1.56 |
0.91 |
1.12 |
0.44 |
| |
P value (rate of weight gain) |
|
<0.001 |
|
|
|
|
|
|
Only significant P values are mentioned. |
TABLE III Proportion of Children Achieving Target Weight at Different Time Intervals
|
Model 1 |
Model 2 |
Model 3 |
Model 4 |
|
Time frame |
RUTF-I (MNT) |
SNT |
Only RUTF-I (MNT) |
DCC |
CMAM |
|
(n=129) |
(n=113) |
(n=355) |
(n=71) |
(n=212) |
|
2 weeks |
25 (19.3%) |
2 (1.8%) |
49 (13.8%) |
32 (45.1%) |
59 (27.9%) |
|
4 weeks |
11 (8.5%) |
8 (7.1%) |
37 (10.4%) |
10 (14.1%) |
24 (11.3%) |
|
6 weeks |
13 (10.0%) |
10 (8.8%) |
32 (9.0%) |
2 (2.8%) |
21 (9.9%) |
|
8 weeks |
9 (6.9%) |
7 (6.2%) |
36 (10.1%) |
7 (9.8%) |
20 (9.4%) |
|
6 months |
20 (15.5%) |
27 (23.9%) |
39 (10.9%) |
4 (5.6%) |
13 (6.1%) |
|
Total |
78 (60.4%) |
54 (47.8%) |
193 (54.3%) |
55 (77.5%) |
137 (64.7%) |
TABLE IV Nutritional Status at 8 Weeks and 6 Months
|
Groups |
Nutritional |
8 wks |
6 mo |
|
|
status |
|
|
|
Model 1 |
RUTF-I
|
n |
76 |
33 |
|
(MNT) |
SAM |
13 (17.1%) |
5 (15.1%) |
|
|
MAM |
32 (42.1%) |
9 (27.3%) |
|
|
Normal |
31 (40.7%) |
19 (57.6%) |
|
SNT |
n |
62 |
36 |
|
|
SAM |
22 (35.4%) |
14 (33.3%) |
|
|
MAM |
28 (45.1%) |
8 (22.2%) |
|
|
Normal |
12 (19.3%) |
16 (44.4%) |
|
Model 2 |
Only RUTF-I
|
n |
200 |
97 |
|
(MNT) |
SAM |
35 (17.5%) |
16 (16.4%) |
|
|
MAM |
90 (43.5%) |
47 (48.4%) |
|
|
Normal |
75 (37.5%) |
34 (36.0%) |
|
Model 3
|
DCC |
n |
77 |
64 |
|
|
SAM |
16 (20.7%) |
11 (17.1%) |
|
|
MAM |
33 (42.8%) |
29 (45.3%) |
|
|
Normal |
28 (36.3%) |
24 (37.5%) |
|
Model 4 |
CMAM |
n |
164 |
58 |
|
|
SAM |
30 (18.3%) |
13 (22.4%) |
|
|
MAM |
72 (43.9%) |
23 (39.6%) |
|
|
Normal |
62 (37.8%) |
22 (37.9%) |
|
SAM: Severe acute malnutrition; MAM: Moderate acute
malnutrition. |
Model 2 (Only RUTF-I)
Three-hundred and fifty-five children completed two
weeks of treatment. The cumulative mean rate of weight gain was 3.25
gm/kg/d during intervention. The mean rate of weight gain was highest in
the initial 14 days (5.67 gm/kg/day) (Table II). A total
of 54.3% children achieved the target weight (Table III).
Only 17.5% and 16.4% children were non-responders at the end of 8 weeks
and 6 months, respectively (Table IV).
Model 3 (Only DCC)
Seventy-one children completed two weeks of
treatment. The cumulative mean rate of weight gain was 5.15 gm/kg/d
during intervention. The mean rate of weight gain was highest in the
initial 14 days (11.14 gm/kg/day) (Table II). A total of
77.5% children achieved the target weight (Table III).
Only 20.7% and 17.1% children were non-responders at the end of 8 weeks
and 6 months, respectively (Table IV).
Model 4 (Only CMAM)
Two-hundred and twelve children completed two weeks
of treatment. The cumulative mean rate of weight gain was 3.2 gm/kg/d
during intervention. The mean rate of weight gain was highest in the
initial 14 days (9.2 gm/kg/day) (Table II). A total of
64.7% children achieved the target weight (Table III).
Only 18.3% and 22.4% children were non-responders at the end of 8 weeks
and 6 months, respectively (Table IV).
Discussion
In this study, the rise in mean weight on initiation
of RUTF-I (MNT) was significantly more rapid as compared to SNT. The
mean rate of weight gain was maximum and statistically significant at 2
weeks in all RUTF-I (MNT) models as compared to the SNT model. It was
maximum for DCC, probably due to the supervised feeding throughout the
intervention period, followed by CMAM and facility-based models. It
decreased steadily over 8 weeks and furthermore till 6 months, but
remained generally higher in the RUTF-I (MNT) group compared with the
SNT group. After the initial rapid weight gain in the first 2 weeks,
there was a plateau effect, which was reflected as the decrease in the
rate of weight gain beyond 2 weeks. This was observed in all four study
models. Target weight was achieved in a larger proportion of children on
RUTF-I (MNT) throughout with statistical significance up to 6 weeks.
Majority of children on RUTF-I (MNT) achieved their target weight in the
first two weeks itself, whereas among children on SNT, majority achieved
their target weight at the end of 6 months. The rate of recovery from
SAM status was higher in all RUTF-I (MNT) groups as compared to SNT
group throughout.
Limitations of our study were: (i) children in
the facility-based model were not supervised daily for RUTF-I (MNT)
consumption after discharge from hospital; (ii) no ration/
financial assistance were provided in SNT group; (iii) the
default rate at 8 weeks was high; (iv) average RUTF-I (MNT)
consumption was less (20%- 60%); and (v) the duration of hospital
stay was not analyzed.
The cumulative mean rate of weight gain in our study
was more than that reported by Cliberto, et al. [7] (2.8 g/kg/d),
but lesser than that reported by other authors [8-10]. Patel, et al.
[11] showed a weight gain of 9 g/kg/day during hospital stay and 3.2
g/kg/day during home-based follow-up, comparable to our study. Recovery
rate at the end of 6 months in RUTF-I (MNT) group (84.8%) was comparable
to 88.5% reported by Gera, et al. [12].
We conclude that RUTF-I (MNT) has an early, rapid and
sustained impact in improvement of nutritional status in a community
setting as well as in a facility-based model.
Acknowledgements: Dean, LTMMC and LTMGH; Toddler
Food Partners - USA; SNEHA; CTARA-IIT-Bombay; UNICEF; and Mr Anil Arekar,
Statistician.
Contributors: AJ: designed the study, .supervised
the trial and contributed to preparation of manuscript; PK: supervised
the trial and wrote the first and final draft of manuscript; LF:
supervised the functioning of the production unit and assisted in final
draft of manuscript; SF: data collection, assisted in statistical
analysis; NS: designed the study trial and setting up of production
unit; MM: designed the study trial and supervised the trial.
Funding: Toddler Food Partners, Minneapolis, USA.
Competing interest: None stated.
|
What is Already Known?
• RUTF is a medical
treatment for Severe Acute Malnutrition recommended by UNICEF
and WHO.
What This Study Adds?
• Indigenously
prepared RUTF is feasible and effective in SAM management, not
only in facility-based but also in community-based care, both in
supervised and unsupervised settings.
|
References
1. "The Indian exception". The Economist. 2011 March
31; Mumbai. Available from: http://www.economist.com/node/18485871.
Accessed February 2, 2018.
2. Golden MH. Evolution of nutritional management of
acute malnutrition. Indian Pediatr. 2010;47:667-78.
3. Shah N, Murty S, Jadhav A, Manglani M, Fernandes
L, Surve A. Indigenous production of ready-to-use therapeutic food to
address severe acute malnutrition in Indian children. Int J Sci Res
Publ. 2015;5:287-94.
4. World Health Organization (WHO) and United Nations
Children’s Fund (UNICEF). WHO Child Growth Standards and the
Identification of Severe Acute Malnutrition in Infants and Children- A
Joint Statement by WHO and UNICEF. 2009. Available from: http://apps.
who.int/iris/bitstream/10665/44129/1/9789241598163_ eng.pdf?ua=1.
Accessed February 2, 2018.
5. Mother and Child Nutrition. Management of Severe
Acute Malnutrition in Children Under Five Years. 2016. Available from:
http://motherchildnutrition.org/mal
nutrition-management/info/appetite-test.html. Accessed February 2,
2018.
6. Ciliberto M, Sandige H, Ndekha MJ, Ashorn P,
Briend A, Ciliberto H, et al. Comparision of home-based therapy
with ready-to-use therapeutic food with standard therapy in the
treatment of malnourished Malawain children: A controlled, clinical
effectiveness trial. Am J Clin Nutr. 2005;81:864-70.
7. Ciliberto MA, Manary MJ, Ndekha MJ, Briend A,
Ashorn P. Home-based theraspy for oedamatous malnutrition with
ready-to-use therapeutic food. Acta Paediatr. 2006;95:1012-5.
8. Diop EHI, Dossou NI, Ndour MM, Briend A, Wade S.
Comparison of the efficacy of a solid ready-to-use food and a liquid,
milk-based diet for the rehabilitation of severely malnourished
children: a randomized trial. Am J Clin Nutr. 2003;78:302-7.
9. Manary M, Ndkeha MJ, Ashorn P, Maleta K, Briend A.
Home based therapy for severe malnutrition with ready-to use food. Arch
Dis Child. 2004;89:557-61.
10. Thakur GS, Singh HP, Patel C. Locally prepared
ready-to-use therapeutic food for children with severe acute
malnutrition: A controlled trial. Indian Pediatr. 2013;50:295-9.
11. Patel D, Gupta P, Shah D, Sethi K. Home-base
rehabilitation of severely malnourished children in resource poor
setting. Indian Pediatr. 2010;47:694-701.
12. Gera T. Efficacy and study of therapeutic
nutrition products for Home based therapeutic nutrition for severe acute
malnutrition: A systematic review. Indian Pediatr. 2010;47:709-18.
13. United Nations Children’s Fund (UNICEF).
Ready-to-use Therapeutic Food for Children with Severe Acute
Malnutrition. 2013. Available from: https://www.unicef.
org/media/files/Position_Paper_Ready-to-use_therap eutic_
food_for_children_with_severe_acute_mal nutrition__ June_2013.pdf.
Accessed February 13, 2018.
14. World Health Organization (WHO) . Guideline
Updates on the Management of Severe Acute Malnutrition in Infants and
Children. 2013. Available from: http://apps.who.int/iris/bitstream/10665/95584/1/9789241506328_eng.pdf.
Accessed February 13, 2018.
15. Golden M, Grellety Y, Schwartz H, Tchibindat F.
Report of a Meeting to Harmonise the Criteria for Monitoring and
Evaluation of the Treatment of Acute Malnutrition in West and Central
Africa. Dakar, Senegal. 30th November – 1st December 2010. Available
from:
http://files.ennonline.net/attachments/1202/consensus-meeting-on-m-e-imam-dakar-2010-eng.pdf.
Accessed February 2, 2018.
16. Singh AS, Kang G, Ramachandran A, Sarkar R, Peter
P, Bose A. Locally made ready-to use therapeutic food for treatment of
malnutrition: A randomized controlled trial. Indian Pediatr.
2010;47:679-86.
17. Linneman Z, Matilsky D, Ndekha M, Maleta K,
Manary MJ. A large-scale operational study of home based therapy with
ready-to-use therapeutic food in childhood malnutrition in Malawi.
Maternal Child Nutr. 2007;3: 206-15.
18. Sachdev HPS, Kapil U, Vir S. Consensus Statement:
National Consensus Workshop on Management of SAM Children through
Medical Nutrition Therapy. Indian Pediatr. 2010;47:661-5.
19. Black RE, Victora CG, Walker SP, Bhutta ZA,
Christian P, de Onis M, et al. Maternal and child undernutrition
and overweight in low-income and Middle income countries. Lancet.
2013;382:427-51.
20. Ashworth A, Khanum S, Jackson A, Schofield C.
Guidelines for the Inpatient Treatment of Severely Malnourished
Children. Geneva: World Health Organization, 2003. Available from:
http://www.who.int/nutrition/publications/guide_inpatient_text.pdf.
Accessed February 2, 2018.
21. Oakley E, Reinking J, Sandige H, Trehan I,
Kennedy G, Maleta K, et al. A ready-to-use therapeutic food
containing 10% milk is less effective than one with 25% milk in the
treatment of severely malnourished children. J Nutr. 2010;140:2248-52.
22. Integrated management of childhood illness:
Caring for Newborns and Children in the Community. Geneva: World Health
Organization; 2011. Available from: http://apps. who.int/iris/bitstream/10665/44398/4/9789241548045_
Chart_Booklet_eng.pdf. Accessed February 2, 2018.
23. Prudhon C, Golden MH, Briend A, Mary JY. A model
to standardize mortality of severely malnourished children using
nutritional status on admission to therapeutic feeding centres. Eur J
Clin Nutr. 1997;51:771-7.
24. Golden MH. The development of concepts of
malnutrition. J Nutr, 2002;132:2117S-22S.
|
|
|
 |
|

