|
|
|
Indian Pediatr 2019;56: 281-286 |
 |
An Optimal Capillary Screen Cut-off of
Thyroid Stimulating Hormone for Diagnosing Congenital
Hypothyroidism: Data from a Pilot Newborn Screening Program in
Delhi
|
|
Prashant Verma 1,
Seema Kapoor1,
Mani Kalaivani2,
Pallavi Vats1,
Sangeeta Yadav1,
Vandana Jain3,
SERB-NBS Initiative Group* and BK Thelma4
From 1Department of Pediatrics, Lok Nayak
Hospital and Maulana Azad Medical College;Departments of 2Biostatistics
and 3Pediatric Endocrinology, All India Institute of Medical
Sciences; and 4Department of Genetics, University of Delhi
(South Campus); New Delhi, India. *Full list of Science and Engineering
Research Board – Newborn Screening Initiative Group (SERB-NBS) members
is provided in Annexure 1.
Correspondence to: Dr Seema Kapoor,
Director-Professor, Department of Pediatrics, Maulana Azad Medical
College, New Delhi, India.
Email: [email protected]
Received: August 11, 2017;
Initial review: January 01, 2018;
Accepted: January 11, 2019.
|
|
Objective: To determine an
appropriate cut-off of capillary Thyroid stimulating hormone (TSH) for
congenital hypothyroidism.
Study design: Cross-sectional.
Participants: 174,000 neonates
born in different hospitals of Delhi, India, from November 2014 to
October 2016.
Main outcome measures:
Correlation between initial and repeat capillary TSH level and
subsequent venous free thyroxine (fT4) level.
Results: 102 newborns with
initial/ repeat capillary TSH level of
³20 mIU/L
(n=174) were confirmed to have congenital hypothyroidism at mean
(SD) age of 5 (4) days. A good correlation between capillary TSH level
and confirmatory venous fT4 level and postnatal age of sampling was
obtained (r -0.6, -0.4). The area under the ROC curve (AUC) was 0.81
(95%CI 0.75 to 0.88), indicating referral capillary TSH level of 20 mIU/L
to be a good predictor of subsequent high venous TSH level.
Conclusion: A cut off of
³20 mIU/L
for capillary TSH screening beyond 24 hours of life is optimal in the
Indian setting for deciding further recall and workup, keeping a balance
between sensitivity and recall rate.
Keywords: Dried blood sample, Evaluation, Free
thyroxine.
|
|
N
eonatal screening programs allow for early
detection and treatment of congenital hypothyroidism (CH), which is the
most common preventable cause of mental subnormality [1,2]. Reported
prevalence of CH has increased over the years due to factors like
detection of milder forms, increasing maternal age, and lower capillary
thyroid stimulating hormone (TSH) cut-offs [3-5]. CH can be either
permanent or transient. Transient CH is a transient abnormality of
thyroid function which reverts later to normal and may or may not
require replacement therapy lifelong. Incidence of transient
hypothyroidism is variable across different regions depending on whether
the condition is defined on the basis of abnormal screen results or
abnormal follow-up confirmatory results at three years of age [6]. Most
centres evaluate the status of children diagnosed as CH in the neonatal
period at 3 years of age for likely withdrawal of therapy. In recent
years, transient neonatal hyperthyrotropinemia, a term applied to those
neonates with abnormal initial transient elevation of neonatal TSH with
normal serum thyroxine (T4) values which reverts to normal at
re-examination within/after two weeks, has gained significance due to
its identification as a risk factor for persistent childhood
hyperthyrotropinemia [7].
In India, where screening is likely to be initiated
across the country with Rashtriya Baal Swasthya Karyakram having CH as a
target disorder; it is important to define specific cut-offs, which can
yield the best sensitivity and specificity and assist in initiation of
empiric therapy. The purpose of this study was to evaluate the
predictive value of various TSH levels. In resource-constrained
settings, it is highly unlikely that one would obtain the results of
venous sample on the same day or early enough on subsequent days.
Methods
A total of 174,000 neonates (94.5% of the total
births) were enrolled between November 2014 and October 2016 from over
20 participating hospitals with a birth cohort of 184,000 births. All
intramural neonates irrespective of gestation, birthweight and admission
to NICU were included in the study. Only those neonates who died within
24 hours of birth, received blood transfusion within 24 hours of birth,
or shifted to another participating hospital were excluded. In preterm
neonates, a second mandated sample was taken at discharge or at two
weeks of postnatal age, whichever was later. The values at repeat sample
in preterm and sick neonates were considered confirmatory after
evaluation of the corresponding venous profile.
Heel prick samples were collected after 24 hours of
birth or at discharge, whichever was later, not later than 36 completed
weeks of gestation in preterm or 14 days of life, whichever was later.
They were dried and transported to a central laboratory of a
tertiary-care teaching hospital located in Delhi. A high TSH with low
free thyroxine (fT4) levels on subsequent venous sample was considered
positive for congenital hypothyroidism. The Institutional Ethics
Committees of all the participating hospitals approved the research
protocol. All the parents or guardians of the infants signed an
individual informed consent.
TSH testing was performed on dried blood samples
(DBS) on 903 S & S GE, Whatmann filter paper using GSP model 2021
(Genetic Screening Processor, Perkin Elmer, Turku, Finland). When the
TSH value on the filter paper was below 10 mIU/L, it was considered
negative and no further action was pursued. Results between 10 and 19.9
mIU/L were considered borderline, and a new DBS was requested usually on
the second or the third day, depending upon whether the patient was in
the hospital or discharged. When the new DBS values were lower than 10
mIU/L, it was considered negative. When the result was still between
10-19.9 mIU/L, venous blood was collected for estimation of fT4 and TSH.
TSH values of 20 mU/L and above on initial filter paper were considered
positive for CH and the newborn was taken up for biochemical and
clinical evaluation immediately. When venous TSH concentration was >10
mU/L and fT4 concentration was <12 pmol/L, the neonate was treated with
L-thyroxine in the dose of 10-15 µg/kg/day and was categorized as
presumptive CH. Newborns with elevated venous TSH and a normal fT4 were
started on L-thyroxine after confirmation of hypothyroidism on thyroid
scan or ultrasound finding of an ectopic or absent gland.
Ultrasonography of the thyroid gland was performed during the first
month of life usually at the time of confirmatory recall. Scintigraphy
of the thyroid was performed prior to initiation of therapy or within 5
days of initiation of therapy.
Statistical analyses: Analyses were performed
using STATA 11 software. Sensitivity, specificity and positive
predictive value was calculated for different neonatal TSH ranges. An
ROC curve was obtained for sensitivity and specificity at different
capillary TSH cut-off levels. Spearman rank correlation was used to
determine correlation between initial screening capillary TSH and venous
fT4 and postnatal age sampling. Mean, median, percentile (0.5-99.5)
capillary TSH values and false positive rates were calculated for
different groups.
Results
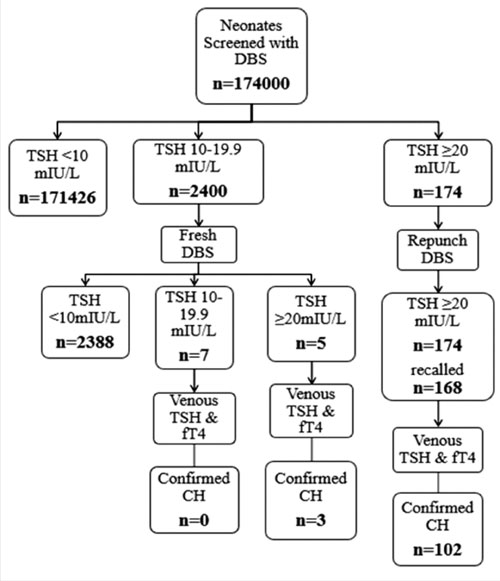
A total of 1,74,000 neonates were screened between
November 2014 to October 2016. TSH levels
³20 mIU/L was found
in 174 neonates, either on initial or repeat capillary screen, out of
which 168 were recalled and evaluated with estimation of fT4, free tri-iodothyronine
(fT3), TSH, thyroid scan and ultrasonography. On follow up, 102 newborns
were confirmed as positive for CH at a mean (SD) age of 5 (4) days, thus
providing an overall prevalence rate of 1 in 1706 screened (Fig.
1). A total of 2400 neonates had values between 10-19.9 mIU/L on
initial capillary screen. Repeat capillary screen of these neonates
identified five with TSH values ³20
mIU/L, and amongst them, three newborns were confirmed to be positive
for CH.
 |
|
Fig. 1 Flow diagram of the study.
|
Of the 102 confirmed cases, 20% (5 of 24) neonates
with capillary TSH in range of 20-29.9 mIU/L had low fT4 levels (<12
pmol/L), while this was 65% (51 of 78) for neonates with capillary TSH
above ³30 mIU/L.
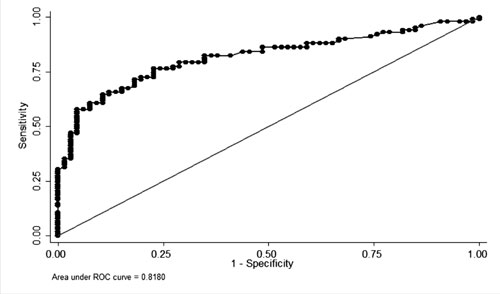
Table I presents diagnostic performance at different TSH cut-off
values. An ROC curve was obtained for sensitivity and specificity at
different capillary TSH cut-off levels. Area under curve (AUC) was 0.81
(95% CI 0.75 to 0.88) (Fig. 2) for a capillary TSH
³20 mlU/L, indicating
referral capillary TSH level to be good predictor of subsequent low
venous TSH level.
TABLE I Diagnostic Performance of Neonatal Capillary TSH Values
|
TSH value (mIU/L)
|
Sensitivity (%) |
Specificity (%) |
PPV (%) |
|
³20 (n=168) |
97 |
99.6 |
61.4 |
|
³30 (n=93) |
76.4 |
99.9 |
83.8 |
|
³40 (n=77) |
66.7 |
99.9 |
88.3 |
|
³50 (n=71) |
62.7 |
100 |
90.1 |
|
TSH: Thyroid stimulating hormone, PPV:Positive predictive value. |
 |
|
Fig. 2 Receiver-operating
characteristic (ROC) curve for capillary TSH value of
³20
mIU/L to predict congenital hypothyroidism.
|
Thyroid scan results were available for 48% of
neonates. Three of the 11 newborns diagnosed as CH with capillary TSH in
the range of 20-29.9 mIU/L had evidence of thyroid dysgenesis on thyroid
scan indicating significant prevalence of hypothyroidism among newborns
with mild elevation in capillary TSH level.
A negative correlation was seen between initial
screening capillary TSH level and venous fT4 level (r= –0.6, P<0.001)
and postnatal age of sampling (r= –0.4, P<0.001).
TABLE II Descriptive Data of Neonates Based on the Postnatal Age
|
Postnatal age of sampling |
|
24-<48 hours
|
48-<72 hours |
72 hours -<7 days
|
≥7 days |
|
Number of neonates (%) |
117116 (67.3) |
45202 (25.9) |
9781 (5.62) |
1901 (1.1)
|
|
Median capillary TSH value |
3.15 |
1.80 |
0.96 |
1.32 |
|
Percentiles (0.5-99.5) |
0.37-14.75 |
0.17-10.56 |
0.08-9.7 |
0.11-9.22 |
|
False positive rate (%) |
50 |
33.3 |
28.6 |
25 |
Capillary TSH values and false positive rates were
higher for newborns with samples collected within 24-<48 hours of birth
(Table II). The 99.5th
percentile value for each group was well below the screening level of 20
mIU/L. Based on this analysis, data was retrospectively analysed with
two sets of age at sampling specific cut- offs. Cut-off 1 was based on
99.9th percentile capillary
TSH values for each group in postnatal age of sampling category as
depicted in Table III. Cut-off 2 was adapted from
published studies with capillary TSH cut off of 34 mIU/L for infants
with 24-<48 hours of age at sampling and value of 28 mIU/L for newborns
with age at sampling of 48 hours [9] .
TABLE III Screening Performance of Individual Cut-offs for Postnatal Age of Sampling
|
Specific cut-off for postnatal age of sampling |
False positive rate (%) |
Recall rate (%) |
Sensitivity (%) |
|
Cut-off 1 |
24-<48 hours |
29 mIU/L |
31.1 |
0.046 |
67.4 |
|
>48 hours |
24 mIU/L |
15.6 |
0.050 |
88.9 |
|
Cut-off 2 |
24-<48 hours |
34 mIU/L |
18.9 |
0.028 |
65.2 |
|
> 48 hours |
28 mIU/L |
10.3 |
0.047 |
89.6 |
|
DBS: dried blood spot, Confirmed CH: TSH >20 mIU/L and fT4 <12
pmol/L. |
Discussion
We report data collected from a large collaborative
study group in Delhi, India,where the prevalance of CH was found to be 1
in 1706. Thyroid dysgenesis was present in significant number of
neonates with confirmed CH. The referral capillary TSH level appears to
be a good predictor of subsequent low venous TSH level. Significant
negative correlation was obtained between initial screening capillary
TSH level and venous fT4 and postnatal age of sampling. Three neonates
with confirmed CH had initial screening capillary TSH of 10-20 mIU/L.
The prevalence in our study is comparable to reports
from various newborn screening programs around the world [1,2]. Recent
studies, including a multi-centric study by Indian Council for Medical
Research, have also reported a higher prevalence of CH in India [10,11].
As seen in this study, an increase in a cut-off of
capillary TSH from 20 mIU/L to 29 mIU/L will lead to an increase in
sensitivity, but many (21 in our study) confirmed cases will be missed.
This underscores the importance of maintaining lower TSH cut-offs to
detect a significant proportion of CH cases. However, further lowering
the cut-off to 10 will lead to an increase in recall rate from 0.1 % to
2%, reducing the efficacy of the program due to low positive predictive
value. As all three confirmed cases with initial TSH between 10-19.9 mIU/L
had a repeat capillary TSH value of
³ 20 mIU/L, repeat sampling and recall is
advisable in only those neonates whose repeat capillary screen levels of
TSH is 10-19.9 mIU/L.
Similar to our findings on significant influence of
postnatal age of sampling on TSH values, other studies have also
suggested the use of age of sampling data in conjunction with absolute
screening capillary TSH values to capture true positive cases [9,12].
Adjustment in cut-off based on postnatal age of sampling in this study
also led to decrease in false positive cases and recall rates; however,
there was a significant decrease in sensitivity with possibility of more
than 15 confirmed cases being missed with both set of cut-offs. Thus,
mild to moderate elevation of capillary TSH above screening level in
newborns with postnatal age of sampling of >48 hours need to be
evaluated more urgently due to lower false positive rates amongst these
groups. Although, delaying postnatal age of sampling will benefit in
terms of decreased recall rate but will lead to increase in overall cost
of program due to increased days of hospital stay. /p>
Across the world, except for some centres in USA and
the Netherlands, most of the newborn screening programs use capillary
TSH levels measured on DBS for screening CH. Cut-off for elevated TSH is
different across various programs with region like Wales (Australia)
using a cut-off as low as 6 mIU/L [13] to a high of 30 mIU/L [14] in
Turkey. Such variations have been attributed to differences in age at
sample collection and the specific type of assay used to measure TSH.
In a report from the Ontario newborn screening
program, 24% newborns were confirmed to have CH with capillary TSH in
the range of 17-29.9 mIU/L [12]. The combined data from 17 Italian
screening centres with low TSH screening cut offs indicated that
approximately 22% of neonates with permanent hypothyroidism were those
who had been identified as a result of the lowered TSH cut off [15].
Studies from China, Iran and Sri Lanka consider capillary TSH of
³20 mIU/L as a strong
indicator for CH with screened newborns being referred immediately for
biochemical and clinical evaluation [16-18]. In a study at a referral
center in Lucknow, initial capillary TSH value between 20-40 mIU/L was
utilized to recall newborns for repeat DBS at 10 days, and later on age
at sampling based cut-off values of >34 mIU/L for 24-48 hours screen and
>20 mIU/L for beyond 48 hours screen were used [10]. According to the
recent recommendations by Indian Society for Pediatric and Adolescent
Endocrino-logy (ISPAE), CH was confirmed and treatment was initiated
when venous confirmatory TSH was >20 mIU/L before the age of 2 weeks and
>10 mIU/L after the age of 2 weeks, with low T4 (<10 µg/dL) [19].
Very low TSH cut-offs (>8 mIU/L), as used by some
newborn screening programs, lead to higher sensitivity but there is
added cost of fairly large number of false positive cases. Krude and
Blankenstein have questioned the benefit of identification of these mild
cases in terms of causing parental distress and higher recall rate [20].
In India, the public health sector is a highly resource- constrained
system with limited provision for dealing with an additional number of
false positive cases and high recall rate.
To conclude, optimal capillary TSH cut off is the
most important determinant for success of any newborn screening program.
Keeping a higher TSH cut-off increases the overall specificity but leads
to significant number of missed cases and very low cut-offs leads to
higher recall rate and cost of program. Thus capillary TSH cut-off of 20
mIU/L as used by our newborn screening program and other newborn
screening programs across different regions can represent a way forward
in this direction. Repeat capillary TSH spot is advised in newborns with
initial TSH value between 10-19.9 mIU/L.
Contributors: PV, SK and BKT: conceived the idea
of research paper; MK: performed the statistical analysis; PV: helped
with study design; SK, SY, PV, SERB-NBS group: managed and followed up
the newborns with congenital hypothyroidism. SK, BKT: conceived the
primary newborn screening project and generated funding support;
SERB-NBS group was involved in data collection and review of manuscript;
VJ, SY, BKT and SK: critically reviewed the manuscript.
Funding: Science and Engineering Research Board,
New Delhi for NBS study in Delhi state vide Grant # IR/SO/LC-0001/2012.
Competing Interest: None stated.
ANNEXURE 1: SERB-NBS Initiative Group Members
MMadhulika Kabra1, Neerja Gupta1,
Ramesh Agarwal1, AK Deorari1, VK Paul1, Shevendru Roy2, RK Sanjeev2, RS
Tomar2, JS Bhasin3, Amit Tyagi3, VK Sharma4 Anil Gulati4, Rajesh Yadav5,
MMA Faridi6, Prerna Batra6, Pooja Dewan6,Veena Devgan7, Alka Mathur7,
Aseem Bhatnagar8, Sunita Bhatia9, Ajay Kumar110, Sushma Nangia10, Arvind
Saili10, Anju Seth10, Deepak Singla11, SK Arora12, S Mehndiratta12,
Ashish Jain13, Gaurav Pradhan13, Sangeeta Gupta13, Siddarth Ramji13,
Mukesh Darshan13, SK Polipalli13, Somesh Kumar13, Biju Varughese13,
Avinash Lomash13, Poonam Sidana14, Sonia Mittal14, Amarjeet Chitkara14,
Arti Maria15, Harish Chellani16, KC Aggarwal16, Shobhna Gupta16, Arya
Sugandha16, Ajay Gambhir17, Surinder Bisht18, Anand Aggarwal19, PM
Kohli19, Indermeet Singh19.
Affiliations: 1All India
Institute of Medical Sciences, 2Army and Base Hospital; 3BLKapoor
Hospital; 4Deen Dayal hospital; 5Girdhari Lal Hospital; 6Guru Teg
Bahadur Hospital; 7Hindu Rao Hospital; 8Institute of Nuclear Medicine
and Allied Sciences; 9Kasturba Hospital; 10Lady Hardinge Medical
College; 11Maharaja Agrasen Hospital; 12Mata Chanan Devi Hospital;
13Maulana Azad Medical College; 14Max Superspeciality Hospital; 15Ram
Manohar Lohia Hospital; 16Safdurjung Hospital & VMM College; 17Saroj
Hospital; 18Swami Dayanand Hospital; and 19Sanjay Gandhi Hospital.
|
What is Already Known?
• There is high prevalence of hypothyroidism in
newborns with mild elevation of capillary thyroid stimulating
hormone (TSH) in newborn screening.
What This Study Adds?
• A cut-off of
³20
mIU/L for capillary TSH screening beyond 24 hours of life is optimal
in the Indian setting.
• Repeat sampling is advised in newborns with
initial TSH value between 10-20 mIU/L.
|
References /p>
1. Grosse SD, Van Vliet G. Prevention of intellectual
disability through screening for congenital hypothyroidism: how much and
at what level? Arch Dis Child. 2011;96:374-9.
2. Olney RS, Grosse SD, Vogt RF Jr. Prevalence of
congenital hypothyroidism–current trends and future directions: Workshop
summary. Pediatrics. 2010;125:S31-6.
3. Rastogi MV, LaFranchi SH. Congenital
hypothyroidism. Orphanet J Rare Dis. 2010;5:17.
4. Harris KB, Pass KA. Increase in congenital
hypothyroidism in New York State and in the United States. Mol Genet
Metab. 2007;91:268-77.
5. Deladoëy J, Ruel J, Giguère Y, Van Vliet G. Is the
incidence of congenital hypothyroidism really increasing? A 20-year
retrospective population-based study in Québec. J Clin Endocrinol Metab.
2011;96:2422-9.
6. Bhavani N. Transient congenital hypothyroidism.
Indian J Endocrinol Metabol. 2011;15:S117-20.
7. Cuestas E, Gaido MI, Capra RH. Transient neonatal
hyperthyrotropinemia is a risk factor for developing persistent
hyperthyrotropinemia in childhood with repercussion on developmental
status. Eur J Endocrinol. 2015;172:483-90.
8. Pokrovska T, Jones J, Shaikh MG, Smith S,
Donaldson MD. How well does the capillary thyroid-stimulating hormone
test for newborn thyroid screening predict the venous free thyroxine
level? Arch Dis Child. 2016;101: 539-45.
9. Lott JA, Sardovia-Iyer M, Speakman KS, Lee KK.
Age-dependent cutoff values in screening newborns for hypothyroidism.
Clin Biochem. 2004;37:791-7.
10. Gopalakrishnan V, Joshi K, Phadke S, Dabadghao P,
Agarwal M, Das V, et al. Newborn screening for congenital
hypothyroidism, galactosemia and biotinidase deficiency in Uttar
Pradesh, India. Indian Pediatr. 2014;51:701-5.
11. Christopher R, Rama Devi AR, Kabra M, Kapoor S,
Mathur R, Muranjan M, et al. Newborn screening for congenital
hypothyroidism and congenital adrenal hyperplasia. Indian J Pediatr.
2018;85:935-40.
12. Saleh DS, Lawrence S, Geraghty MT, Gallego PH,
McAssey K, Wherrett DK, et al. Prediction of congenital
hypothyroidism based on initial screening thyroid-stimulating-hormone.
BMC Pediatr. 2016;16:24.
13. Pryce RA, Gregory JW, Warner JT, John R, Bradley
D, Evans C. Is the current threshold level for screening for congenital
hypothyroidism too high? An audit of the clinical evaluation,
confirmatory diagnostic tests and treatment of infants with increased
blood spot thyroid-stimulating hormone concentrations identified on
newborn blood spot screening in Wales. Arch Dis Child. 2007;92:1048.
14. Büyükgebiz A. Newborn screening for congenital
hypothyroidism. J Pediatr Endocrinol Metab. 2006;19: 1291-8.
15. Olivieri A, Corbetta C, Weber G, Vigone MC,
Fazzini C, Medda E; Italian Study Group for Congenital Hypothyroidism.
Congenital hypothyroidism due to defects of thyroid development and mild
increase of TSH at screening: Data from the Italian National Registry of
infants with congenital hypothyroidism. J Clin Endocrinol Metab.
2013;98:1403-8.
16. Zhao DH, Shen Y, Gong JM, Meng Y, Su L, Zhang X.
Newborn screening for congenital hypothyroidism in Henan province,
China. Clin Chim Acta. 2016;452:58-60.
17. Dorreh F, Chaijan PY, Javaheri J, Zeinalzadeh AH.
Epidemiology of congenital hypothyroidism in Markazi Province, Iran. J
Clin Res Pediatr Endocrinol. 2014;6: 105-10.
18. Lucas G. Guidelines on Management of Congenital
Hypothyroidism in Sri Lanka. Sri Lanka J Child Health. 2015;44:75-6.
19. Desai MP, Sharma R, Riaz I, Sudhanshu S, Parikh
R, Bhatia V. Newborn screening guidelines for congenital hypothyroidism
in India: Recommendations of the Indian Society for Pediatric and
Adolescent Endocrinology (ISPAE) - Part I: Screening and Confirmation of
Diagnosis. Indian J Pediatr. 2018;85:440-7.
20. Krude H, Blankenstein O. Treating patients not
numbers: The benefit and burden of lowering TSH newborn screening
cut-offs. Arch Dis Child. 2011;96:121-2.
|
|
|
 |
|

