|
|
|
Indian Pediatr 2018;55:307-310 |
 |
Vitamin D Deficiency in
Ambulant Children on Carbamazepine or Sodium Valproate
Monotherapy
|
|
Mini Sreedharan 1,
Kalpana Devadathan1,
PA Mohammed Kunju1,
Bindusha Sasidharan2,
Jayakumar Parameswaran Pillai3,
Minikumari Amma Vasumathy Amma3
and Saboorabeegum MuthuBeevi3
From Departments of 1Pediatric Neurology,
2Pediatrics, and 3Biochemistry; Government Medical
College, Thiruvananthapuram, India.
Correspondence to: Dr Kalpana Devadathan, Additional
Professor, Department of Pediatric Neurology, Government Medical
College, Thiruvananthapuram, Kerala, India.
Email:
[email protected]
Received: May 17, 2017;
Initial review: July 03,2017;
Accepted: January 03, 2018.
Published online:
February 09, 2018.
PII:S097475591600117
|
Objective: To assess the effect of monotherapy with Carbamazepine
(CBZ) and Sodium valproate (VPA) on serum 25-OH vitamin D levels in
children with epilepsy compared to controls.
Design: Cross-sectional study.
Setting: Outpatient department of a tertiary-care
Pediatric Neurology centre, and a nearby day-care centre and school.
Study period: June 2012 to May 2013
Participants: Children with epilepsy aged 2 to 13
years on monotherapy with CBZ (n=28) or VPA (n=28) for at
least 6 months; 109 age-matched controls from a nearby day-care centre
and school.
Results: The median (IQR) values of 25 (OH)
vitamin D was 18.0 ng/mL (13.7-27.3), 21.35 ng/mL (16.4 -25.2) and 30.5
ng/mL (19.1-43.7) in CBZ, VPA and control group, respectively (P=
0.008). 60.7% of patients in CBZ group and 35.7 % in VPA group had low
25 (OH) D levels (<20 ng/mL) compared to 27.8% in controls (P=0.001).The
serum alkaline phosphatase level was higher in children on carbamazepine
therapy (P=0.001) than controls.
Conclusion: This study identifies significant
risk of vitamin D deficiency in ambulant children with epilepsy on
monotherapy with CBZ or VPA.
Keywords: Adverse effect; Antiepileptic drugs; Hypo-
vitaminosis D.
|
|
B
iochemical abnormalities of bone mineral
metabolism in children receiving antiepileptic drugs, first identified
in 1979 [1], is still a poorly studied topic from this region. In India,
it is not a routine practice to supplement calcium or vitamin D in
children on antiepileptic drugs; even in the UK, only 3% of Pediatric
neurologists were reported to be using prophylactic calcium and vitamin
D therapy for children on anticonvulsants [2]. Available evidence
indicates that vitamin D levels in the Indian population is below the
optimal levels recommended by the US Institute of Medicine or US
Endocrinology Society [3]. Majority of previous studies included
children on polytherapy, institutionalized children or those with
cerebral palsy who were indoors most of the time and from geographic
areas with less sunshine, all of which are independent risk factors for
low vitamin D levels. We planned this study to assess the effect of
monotherapy with two most commonly used AEDs, CBZ and VPA, on bone
mineral metabolism in ambulatory children with epilepsy, with normal
physical and mental development.
Methods
Kerala is a state located at the southern tip of
India and receives adequate sunshine throughout the year. Consecutive
ambulant children (aged 2-13 years) with epilepsy and having apparently
normal physical and mental development, and attending the Pediatric
Neurology outpatient department of a tertiary referral hospital in
Kerala between June 2012 and May 2013, on either CBZ or VPA monotherapy
for at least six months, were included in the study after taking
informed consent. The study protocol was approved by the Institutional
Research Board and ethical clearance was granted by the Institutional
Ethical Committee. Children who received vitamin D or calcium
supplementation, those on polytherapy with antiepileptic drugs (AEDs) or
any chronic medications likely to affect bone metabolism like vitamin A,
anabolic steroids, bisphosphonates, gluco-corticoids, thiazides,
calcitonin etc. and children with history of malabsorption,
hypothyroidism, hepatic or renal diseases were excluded from the study.
Control group included age-matched children attending a nearby day-care
centre and a school, who were not on any continuous medications during
the same period of study.
Demographic data including age, weight, height, BMI,
average duration of exposure to sunlight per day, type of epilepsy,
drugs used for treatment of epilepsy, duration of epilepsy, frequency of
seizures per month, type of epilepsy, and duration of antiepileptic
therapy were collected. Serum calcium, phosphorus, alkaline phosphatase,
proteins, urea, creatinine, aspartate amino transferase (AST), alanine
amino transferase (ALT), fasting lipid profile and 25-hydroxy vitamin D
[25 (OH) D] levels were assessed. Serum was separated by centrifuging at
room temperature and then stored at –20 º
C until vitamin D analysis was performed.
25 (OH) D level was analyzed using ELISA 96T kit
(Diametra, Italy) within two weeks of sample collection. The lowest
detectable concentration of 25 OH vitamin D is 0.3 ng/mL at 95%
confidence limit. The intra assay variability was less than 6.4% and
inter-assay variability was less than 6.95% (precision values as per the
manufacturer). Children with serum level of 25 (OH) D level of more than
20 ng/mL (50 nmol/L) was considered sufficient, levels between 12-20 ng/mL
(30-50 nmol/L) were considered insufficient, and below 12 ng/mL (<30
nmol/L) were considered deficient [4].
A priori power calculation based on a previous
study [5] indicated that a sample size of 30 children in each group
would provide 80% power to detect a 10% difference in 25(OH) D
concentration, using a two-tailed t-test, while controlling type I error
rate to 5%.
Statistical analysis: Comparison of quantitative
data between two groups were analyzed by independent sample t test or
Mann Whitney U test according to the nature of the data. Comparison of
quantitative data among more than two groups were analysed by ANOVA with
post hoc analysis or Kruskal Wallis test. Association between
qualitative data were analyzed by Chi-square test. A P value of
<0.05 was taken as statistically significant. Data analysis was
performed using SPSS version 22.0.
Results
56 children with epilepsy (28 each receiving CBZ and
VPA monotherapy) and 109 controls were enrolled in the study. None of
the cases or controls had fractures, bone pain, muscle pain or muscle
weakness. Clinical features of rickets were absent in both cases and
controls (Table I). Serum albumin and protein levels were
in the normal range for all the three groups.
TABLE I Demographic Characteristics of Children Receiving Antiepileptic Drugs and Controls
|
Variable |
CBZ (n= 28) |
VPA (n=28) |
Control (n=109) |
P |
|
Age (y) |
8.0 (2.9) |
8.2 (2.9) |
8.9 (2.2) |
0.17 |
|
Male gender, n (%) |
15 (53.6) |
13 (46.4) |
62 (56.9) |
0.61 |
|
Weight (kg) |
23.2 (7.37) |
25.2 (10.49) |
27.7 (9.9) |
0.06 |
|
Height (cm) |
124.3 (15.95) |
126.7 (18.23) |
129. 7 (12.0) |
0.86 |
|
BMI (Kg/ m2 ) |
14.7 (2.4) |
15.1 (2.8) |
16.2 (4.0) |
0.09 |
|
Exposure to direct sunlight (hr/wk) |
2.8 (2.1) |
2.7(1.6) |
3.01(1.83) |
0.61 |
|
Duration of epilepsy |
23.4 |
24.9 |
- |
0.79 |
|
Duration of AED therapy (months) |
23.4 (6-86) |
24.9 (6-84) |
- |
0.78 |
|
All values in mean (SD); AED: Antiepileptic drugs; BMI: Body
mass index; CBZ: Carbamazepine; VPA: Sodium valproate. |
 |
|
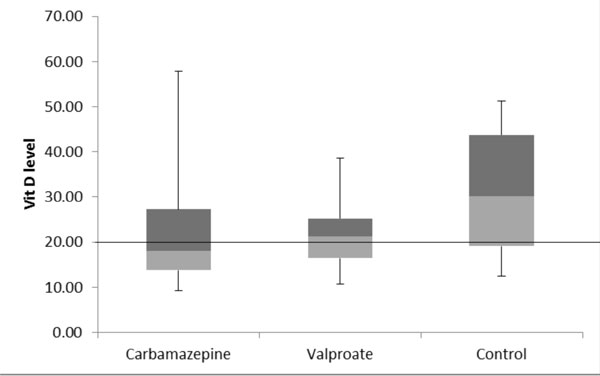
Fig. 1 Box and whisker plot showing
vitamin D levels in children on valproate Or carbamazepine
monotherapy.
|
The median values of 25 (OH) vitamin D were 18.0
ng/mL (IQR 13.7-27.3), 21.3 ng/mL (IQR 16.4 -25.2) and 30.1 ng/mL (IQR
19.1-43.7) in the CBZ , VPA and control group, respectively (P=0.008).
Comparison between CBZ and Controls (P=0.01) and VPA and Controls
(P=0.02) also showed significant differences (Fig. 1).
The proportion of participants with subnormal vitamin D levels (<20
ng/mL) were significantly different between the groups (Table
II). Alkaline phosphatase levels were significantly higher among
children on CBZ compared to controls (P=0.001) (Table
III).
TABLE II Vitamin D Status of Cases and Controls
|
Drug |
Vitamin D (ng/mL), no (%) |
|
<12 (n=17) |
12-20 (n=40) |
>20 (n=108) |
|
CBZ |
5 (17.9) |
12 (42.9) |
11 (39.3) |
|
VPA |
4 (14.3) |
6 (21.4) |
18 (64.3) |
|
Controls |
8 (7.3) |
22 (20.2) |
79 (72.5) |
|
P=0.02; (<12 ng/mL – Deficient, 12-20 ng/mL Insufficient,
>20 ng/mL- Sufficient) [4], CBZ: Carbamazepine; VPA: Sodium
valproate. |
TABLE III Calcium, Phosphorus and Alkaline Phosphatase Levels in Those Receiving Antiepileptic Drugs and Controls
|
Carbamazepine (n=28) |
Valproate (n=28) |
Control (n=109) |
P
|
|
Calcium (mg/dL)
|
9.6 (0.6) |
10.2 (0.8) |
9.6 (1.4) |
0.026 |
|
Phosphorus (mg/dL)
|
4.8 (0.6) |
5.8 (5.2) |
4.5 (0.7) |
0.031 |
|
Alkaline phosphatase (IU/L)
|
267.8 (72.3) |
191.2 (52.3) |
219.2 (68.4) |
<0.001 |
|
All values in mean (SD). |
Discussion
This hospital-based cross-sectional study found a
significantly high proportion of children receiving AEDs to have
hypovitaminosis D, as compared to controls.
The strengths of our study include the strict
selection criteria, exclusion of bed-ridden subjects and children on
polytherapy with AEDs, and a large number of healthy control children.
Lack of exposure to natural sunlight and osteoporosis following
inactivity are two well-known contributors of osteoporosis. Some of the
previous studies included significant percentage of children with
cerebral palsy and on polytherapy [6].
Borusiak, et al. [7] found significant
hypocalcemia and low levels of vitamin D among 128 ambulant children on
multiple antiepileptic drugs. As all the children in our study were on
monotherapy, the alteration in various parameters can be attributed to
the drug itself. The one important limitation of our study is the
cross-sectional design; a longitudinal follow up of these children might
have been a more accurate reflection of bone health in these children.
Lee, et al. [8] longitudinally followed up children with epilepsy
on antiepileptic drugs and found that a high proportion of children had
hypovitaminosis D before the start of treatment, and a significant
decrease in levels was noted between the initial and the follow up after
6 months [8]. This suggested epilepsy as a risk factor for vitamin D
deficiency, which will be augmented by antiepileptic drugs. We did not
attempt the bone mineral density estimation by DEXA scan, which
accurately reflects the bone health, because of financial constraints
and the risk of exposure to X-ray irradiation. Other parameters
like osteocalcin levels, serum parathormone levels and calcitonin levels
were also not assayed.
Fong, et al. [9] found that Indian ethnicity,
immobility and polytherapy with AEDs were significant risk factors for
low vitamin D levels in children with epilepsy. Seth, et al. [10]
found that 83% of non-ambulant children with cerebral palsy on
antiepileptic drugs were vitamin D deficient. Other authors have also
reported low vitamin D levels in adults and children [11,12]. However,
Turan, et al. [13] noted that CBZ, VPA and phenobarbitone therapy
did not show any effect on serum vitamin D levels, as also reported with
VPA in another study [14]. Hepatic induction of the cytochrome P450
enzyme system leading to increased catabolism of vitamin D is the
principal mechanism reported in case of enzyme-inducing drugs like
Carbamazepine [15]. Valproate inhibits the 25-hydroxylase activity on
vitamin D in liver mitochondria without inhibiting the components of
cytochrome P450-linked mono-oxygenase systems [16]. It is proposed that
genetic variations like polymorphisms in vitamin D receptor (VDR) gene
may predispose one to vitamin D deficiency [17]. The significant
increase in serum alkaline phosphatase in children on carbamazepine may
be attributed to changes in bone mineral metabolism due to its enzyme
inducing property [18]. Mikati, et al. [19] studied the effect of
low dose vs high dose vitamin D in ambulatory adults and children
on antiepileptic drugs and found that high-dose vitamin D therapy
substantially increased bone mineral density at several skeletal sites
in adults. In children, both doses resulted in comparable increases in
bone mass.
This study shows that serum 25 OH vitamin D levels
are significantly low in children on carbamazepine or valproate
monotherapy. Children on antiepileptic drugs should have regular
monitoring of Vitamin D levels, and/or supplementation with calcium and
vitamin D even in children with normal growth and development, no
limitation of physical activity and adequate exposure to sunshine. The
impact of antiepileptic drugs on bone health is to be addressed by all
Pediatricians, as early identification of vitamin D deficiency and
supplementation of calcium and vitamin D can help majority of children
on long term anticonvulsants.
Contributors: MS,KD: conceptualised the idea,
design, collection of data, analysis, preparation of manuscript, review
of literature; PAMK: supervised the study and edited the manuscript; BA:
collection and analysis of data of the control population; JP:
statistical analysis and preparation of tables and figures; MV:
biochemical analysis and interpretation of data; SB: supervised the
biochemical analysis.
Funding: A research grant from SAT Hospital
endowment fund. Competing interest: None stated.
|
What is Already Known?
•
Vitamin D deficiency is common
in children with developmental delay on multiple antiepileptic
drugs.
What This Study Adds?
•
Vitamin D deficiency is also seen in typically developing
children on monotherapy with either Carbamazepine or Sodium
valproate monotherapy.
|
References
1. Offermann G, Pinto V, Kruse R. Antiepileptic
drugs and vitamin D supplementation. Epilepsia. 1979; 20:3-15.
2. Fong CY, Mallick AA, Buren CP, Patel JS.
Evaluation and management of bone health in children with epilepsy
on long-term antiepileptic drugs: United Kingdom survey of
paediatric neurologists. Eur J Paediatr Neurol. 2011; 15:417-23.
3. Holick MF, Binkley NC, Bischoff-Ferrari HA,
Gordon CM, Hanley DA, Heaney RP, et al; Endocrine Society.
Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An
Endocrine Society Clinical Practice Guideline. J Clin Endocrinol
Metab. 2011;96:1911-30.
4. Munns CF, Shaw N, Kiely M, Specker BL, Thacher
TD, Ozono K, et al. Global Consensus Recommendations on
Prevention and Management of Nutritional Rickets. Horm Res Paediatr.
2016; 85:83-106.
5. Ramelli V, Ramelli GP, Lava SA, Siegenthaler
GM, Cantù M, Bianchetti MG, et al. Vitamin D status among
children and adolescents on anticonvulsant drugs in Southern
Switzerland. Swiss Med Wkly. 2014; 13:w13996.
6. Coppola G, Fortunato D, Auricchio G, Mainolfi
C, Operto FF, Pascotto A, et al. Bone mineral density in
children, adolescents, and young adults with epilepsy. Epilepsia.
2009;50:2140-6.
7. Borusiak P, Langer T, Heruth M, Karenfort M,
Bettendorf U, Jenke AC. Antiepileptic drugs and bone metabolism in
children: Data from 128 patients. J Child Neurol. 2013;28:176-83.
8. Lee YJ, Park KM, Kim YM, Yeon GM, Nam SO.
Longitudinal change of vitamin D status in children with epilepsy on
antiepileptic drugs: prevalence and risk factors. Pediatr Neurol.
2015;52:153-9.
9. Fong CY, Kong AN, Poh BK, Mohamed AR, Khoo TB,
Ng RL, et al. Vitamin D deficiency and its risk factors in
Malaysian children with epilepsy. Epilepsia. 2016;57:1271-9.
10. Seth A, Aneja S, Singh R, Majumdar R, Sharma
N, Gopinath M. Effect of impaired ambulation and anti-epileptic drug
intake on vitamin D status of children with cerebral palsy. Paediatr
Int Child Health. 2017;37:193-8.
11. Misra A, Aggarwal A, Singh O, Sharma S.
Effect of carbamazepine therapy on vitamin D and parathormone in
epileptic children. Pediatr Neurol. 2010; 43:320-4.
12. Menon B, Harinarayan CV. The effect of
anti-epileptic drug therapy on serum 25-hydroxyvitamin D and
parameters of calcium and bone metabolism – A longitudinal study.
Seizure. 2010;19:153-8.
13. Turan MI, Cayir A, Ozden O, Tan H. An
examination of the mutual effects of valproic acid, carbamazepine,
and phenobarbital on 25-hydroxyvitamin D levels and thyroid function
tests. Neuropediatrics. 2014;45:16-21.
14. Rauchenzauner M, Griesmacher A, Tatarczyk T,
Haberlandt E, Strasak A, Zimmerhackl LB, et al. Chronic
antiepileptic monotherapy, bone metabolism, and body composition in
non-institutionalized children. Dev Med Child Neurol. 2010;52:283-8.
15. Pack AM, Morrell MJ. Adverse effects of
antiepileptic drugs on bone structure: epidemiology, mechanisms and
therapeutic implications. CNS Drugs. 2001;15:633-42.
16. Tomita S, Ohnishi J, Nakano M, Ichikawa Y.
The effects of anticonvulsant drugs on vitamin D3-activating
cytochrome P-450-linked monooxygenase systems. J Steroid Biochem Mol
Biol.1991;39:479-85.
17. Uitterlinden AG, Fang Y, Bergink AP, van
Meurs JB, van Leeuwen HP, Pols HA. The role of vitamin D receptor
gene polymorphisms in bone biology. Mol Cell Endocrinol.
2002;197:15-21.
18. Coppola G, Fortunato D, Auricchio G, Mainolfi
C, Operto FF, Pascotto A, et al. Bone mineral density in
children, adolescents, and young adults with epilepsy. Epilepsia.
2009;50:2140-6.
19. Mikati MA, Dib L, Yamout B, Sawaya R, Rahi AC, Fuleihan Gel-H.
Two randomized vitamin D trials in ambulatory patients on
anticonvulsants: Impact on bone. Neurology. 2006;67:2005-11.
|
|
|
 |
|

