|
|
|
Indian Pediatr 2018;55: 301-305 |
 |
Survival of Children
Living with Human Immunodeficiency Virus on Antiretroviral
Therapy in Andhra Pradesh, India
|
|
Ugra Mohan Jha 1,
Neeraj Dhingra1,
Yujwal Raj2,
Bharat Bhusan Rewari1,
L Jeyaseelan3,
Pauline Harvey4,
Laxmikant Chavan5,
Niranjan Saggurti6
and DCS Reddy2
From 1National AIDS Control Organization,
New Delhi; 2Independent consultants, New Delhi; 3Christian
Medical College, Vellore; 4Center for Disease Control and
Prevention, Global Health-India, New Delhi; 5World Health
Organization, New Delhi; and 6Bill and Melinda Gates
Foundation, New Delhi; India.
Correspondence to: Ugra Mohan Jha, National AIDS
Control Organization, New Delhi, India.
Email: [email protected]
Received: May 24, 2016;
Initial review: August 31, 2016;
Accepted: January 23, 2018.
Published online:
February 09, 2018.
PII:S097475591600107
|
|
Objectives: To assess the survival probability and associated
factors among children living with human immunodeficiency virus (CLHIV)
receiving antiretroviral therapy (ART) in India.
Methods: The data on 5874
children (55% boys) from one of the high HIV burden states of India from
the cohort were analyzed. Data were extracted from the computerized
management information system of the National AIDS Control Organization
(NACO). Children were eligible for inclusion if they had started ART
during 2007-2013, and had at least one potential follow-up. Kaplan Meier
survival and Cox proportional hazards models were used to measure
survival probability.
Results: The baseline median
(IQR) CD4 count at the start of antiretroviral therapy was 244 (153,
398). Overall, the mortality was 30 per 1000 child years; 39 in the <5
year age group and 25 in 5-9 year age group. Mortality was highest among
infants (86 per 1000 child years). Those with CD4 count
³200
were six times more likely to die (adjusted HR: 6.3, 95% CI 3.5, 11.4)
as compared to those with a CD4 count of
³350/mm3.
Conclusion: Mortality rates
among CLHIV is significantly higher among children less than five years
when the CD4 count at the start of ART is above 200. Additionally, lower
CD4 count, HIV clinical staging IV, and lack of functional status seems
to be associated with high mortality in children who are on ART.
Keywords: HIV, Mortality, Outcome,
Predictors, Treatment.
|
|
I
ndia has witnessed a decline in mortality among
people (including children) living with human immunodeficiency virus
(HIV) who are on antiretroviral therapy (ART), during 2007-2011 [1].
This decline in mortality is argued to be attributed to the scale-up of
ART in the country [2]. Globally, half the Children living with HIV
(CLHIV) die of Acquired immune deficiency syndrome (AIDS) before their
second birthday, and one-third during infancy in the absence of ART [3].
On the other hand, ART coverage for CLHIV had been scaled up
significantly across the country during 2007 to 2013 [2]. Studies that
examine the mortality/survival status of children who are on ART are
scarce in India.
The National AIDS Control Organisation (NACO)
revealed an overall decline in people dying of AIDS related causes
during 2007-2013; however, not much improvement was noted for children.
This study was, therefore, conducted to assess the mortality among CLHIV
receiving ART during 2007-2013, and the factors influencing the same.
Methods
We analyzed records of 5874 CLHIV under 15 years of
age, who had been initiated on ART between January 2007 and December
2013. Data were obtained from 45 ART centres across 23 districts in high
HIV prevalence settings (combined Andhra Pradesh and Telangana) in
India. These ART centres were set up at different points of time during
2007 to 2013. The patients’ ART identification numbers were used to
extract information from the patients’ records entered in electronic
Computerized Management Information System Software (CMIS). The
information on socio-demographic characteristics, baseline clinical and
laboratory measurement, and treatment outcomes were extracted from the
database, and the primary outcome measure of patient mortality (time
death) was noted.
Data were analyzed using the Kaplan Meier survival
and Cox proportional hazard model to measure survival and identify
independent predictors of mortality of CLHIVs on ART. Cox proportional
hazard ratios (HR) and adjusted hazard ratios (AHR) with 95% confidence
intervals were used to assess the effect of baseline predictors on the
survival of children on ART. Key variables used in the analysis
included: gender, educational status (no schooling, attended school,
information missing), CD4 count at ART initiation ( £
200, 201-250, 251-350, ³350),
age at registration, age at start of ART (0-4 years, 5-9 years, 10-14
years), follow-up at most recent visit, CD4 count at ART initiation, WHO
clinical stage (I, II, III, IV) and functional status (ambulatory, bed
ridden and functional).
This study follows Cox regression model fulfilling
the assumption of "non-informative censoring". The incidence rates of
mortality, the primary end point of interest, were calculated by
dividing the number of deaths by the total number of person-years. For
each member of the cohort, person-years at risk were measured from the
start date of ART until the date of the most recent clinic visit.
Kaplan-Meier methods were used to assess the cumulative probability of
survival after the start of ART. For analysis and compilation of data
SPSS 20.0, STATA 12.0 and MS Excel were used.
Results
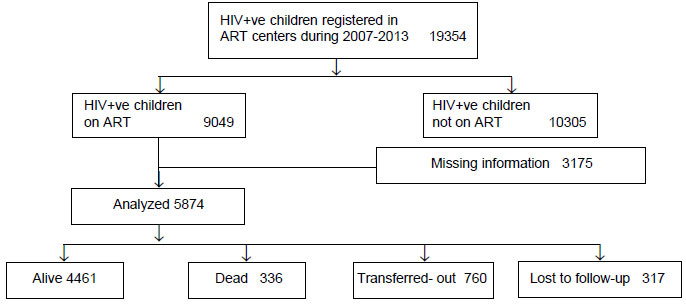
Of the 5874 children who were included in the
analyses, 4461 (76%) children were alive, 336 (6%) died, and 760 (13%)
were transferred out of the facility over the study period (Fig.
1). Table I depicts the characteristics of child-ren
included in the study. The median (IQR) age at the start of ART was 8
(5,11) years. The median (IQR) CD4 count at the start of ART was 244
(153, 398)/mm 3. The
age-specific mortality rate among children on ART was highest among
children younger than 5 years (Table II).
 |
|
Fig. 1 Flow diagram of distribution
of HIV +ve children included in the study.
|
TABLE I Characteristics of Children on Antiretroviral Therapy (N=5874)
|
Variables |
Number (%) |
|
Male gender |
3245 (55.2) |
|
Age group |
|
0-4 y |
967 (16.5) |
|
5-9 y |
2558 (43.5) |
|
10-14 y |
2349 (40) |
|
CD4 count at start of ART (n=4843) |
|
≤200 |
1814 (37.5) |
|
201-250 |
680 (14) |
|
251 – 350 |
970 (20) |
|
351+ |
1379 (28.5) |
|
HIV clinical stage (n=4981) |
|
I+II |
3354 (57.1) |
|
III |
1398 (23.8) |
|
IV |
229 (3.9) |
|
Functional status (n=4991) |
|
Ambulatory |
316 (6.3) |
|
Bedridden |
65 (1.3) |
|
Functional |
4610 (92.4) |
|
Treatment outcomes |
|
Alive |
4461 (75.9) |
|
Died |
336 (5.7) |
|
Transfer out |
760 (12.9) |
|
Lost to follow-up |
317 (5.4) |
TABLE II Age-specific Mortality Rates Among Children in Present Study
|
Age group |
Number at the |
Number of |
Number of |
Total child- |
Incidence mortality rates |
CD4 count (per mm3) |
|
start of ART |
LFU |
deaths |
years |
(per 1000 person years) |
Median (IQR) |
|
0 – 4 y |
967 |
65 |
73 |
1884 |
39 |
341 (195,766) |
|
5 – 9 y |
2558 |
108 |
132 |
5357 |
25 |
240 (155,383) |
|
10 – 14 y |
2349 |
144 |
131 |
4107 |
32 |
227 (139, 336) |
|
Total |
5874 |
317 |
336 |
11348 |
30 |
244 (153, 398) |
|
ART: anti-retroviral therapy; IQR: inter quartile range;
LFU: Lost to follow-up. |
Children with CD4 count
£200 were six times
more likely to die (Adjusted Hazard Risk Ratio (AHR): 6.3; 95% CI
3.5-11.4) as compared to those with a higher CD4 count (³350)
(Table III). Children who were in HIV clinical stage IV
were three times more likely to die (AHR 3.2, 95% CI 1.9-5.3) as
compared to those in the clinical stage I and II. Bedridden children
were 4.5 times more likely to die (AHR 4.5; 95% CI 2.2-9.4) as compared
to the children who were functional (Table III). There was
no interaction effect between the child’s age and CD4 count. Baseline
CD4 count had independent risk and the incidence was around 90, which is
3 times higher than the overall incidence density of 30. In the category
with CD4 count 201-250, age (under 5 years) was a significant predictor,
with mortality of 100 per 1000 person years, which was more than 3 times
higher than the overall mortality of 30 per 1000 person years and also
two and half times that (HR 2.621, 95% CI 1.05-6.52) observed in the
10-14 years age-group (P<0.05).
TABLE III Factors Associated with Mortality Among Children on ART (N=5874)
|
Characteristics |
Censored |
Dead |
Unadjusted Hazard ratio |
Adjusted Hazard ratio |
|
|
|
(95% CI) |
(95% CI) |
|
Age at ART initiation |
|
0 – 4 years |
894 |
73 |
1.34 (1.01, 1.78)# |
0.48 (0.22, 1.08) |
|
5 – 9 years |
2426 |
132 |
0.88 (0.68, 1.12) |
0.84 (0.62, 1.14) |
|
10 – 14 years |
2218 |
131 |
Reference |
Reference |
|
Gender |
|
|
|
|
|
Male |
3050 |
195 |
1.12 (.90-1.39) |
1.20 (0.90,1.62) |
|
Female |
2488 |
141 |
Reference |
Reference |
|
CD4 count at ART initiation |
|
≤ 200 |
1656 |
158 |
4.45 (2.29, 6.62)‡ |
6.27 (3.46, 11.36)‡ |
|
201-250 |
647 |
33 |
2.48 (1.51, 4.09)‡ |
2.97 (1.45, 6.09)‡ |
|
251 – 350 |
927 |
43 |
2.32 (1.45, 3.72)‡ |
3.241 (1.66, 6.32‡ |
|
351+ |
1350 |
29 |
Reference |
Reference |
|
WHO clinical stage |
|
I/II |
3218 |
136 |
Reference |
Reference |
|
III |
1293 |
105 |
1.82 (1.41, 2.34)‡ |
1.38 (1.01, 1.91)# |
|
IV |
188 |
41 |
4.89 (3.45, 6.95)‡ |
3.18 (1.92, 5.28 |
|
Functional status |
|
|
|
|
|
Ambulatory |
272 |
44 |
3.13 (2.27, 4.34)‡ |
2.263 (1.44, 3.55)‡ |
|
Bed ridden |
41 |
24 |
10.06 (6.60, 15-36)‡ |
4.51 (2.17, 9.36)‡ |
|
Functional |
4385 |
225 |
Reference |
Reference |
|
#P<0.05; ‡P<0.001. |
Out of those children who died, 37% died within a
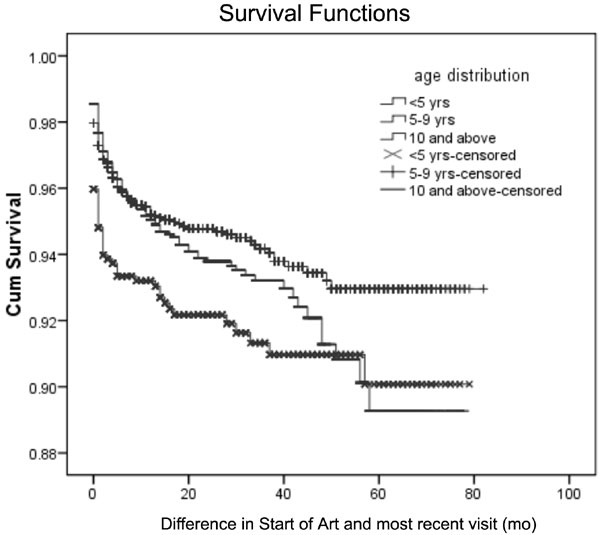
month of starting ART, and 74% by six months. The overall estimated
cumulative survival probability was 0.948 (95% CI 0.94-0.95) after 12
months, and 0.911 (95% CI 0.89-0.92) after 60 months. In the younger (<5
years) age group, cumulative survival probability at 12 months was 0.929
(95% CI 0.91-0.94) and 0.897 (95% CI 0.86-0.92) after 60 months. In the
older age group (10 years and above), the cumulative survival
probability after 12 months was 0.949 (95% CI 0.93-0.95) and after 60
months was 0.889 (95% CI 0.85-0.91) (Fig. 2).
 |
|
Fig. 2 Survival curves of
HIV-positive children by age.
|
Discussion
Our findings indicate high levels of mortality among
CLHIV. The incidence mortality rates are marginally higher amongst the
youngest children (less than five years) than those among the older
children. The corresponding probability of survival among children
living with HIV is 95% after 12 months follow-up and 91% after 60-month
from the start of their ART initiation. Results further suggest that CD4
count at ART initiation, WHO clinical staging and functional status of
children at the time of baseline seems to independently determine the
subsequent survival status of the children.
These results are comparable with earlier research
from Gujarat (India) that suggested similar (but lower) survival
probability of 86% after 36 months from the start of ART among children
[10]. This suggests an improvement in child survival over time in India.
The child mortality (30 per 1000 child years) in the current study found
to be lower when compared to two different cohort studies conducted in
Kenya which reported an overall mortality as 47 and 84 deaths per 1000
child years) [6,7]. The timing of deaths after start of ARI in this
study is consistent with study results from northwest Ethiopia which
showed that majority (90%) of the deaths occurred within the first year
of treatment, and almost 50% within the first month [3,4,8].
The present study findings are important given the
paucity of literature documenting the survival status of children who
are on ART in India. However, the results must be considered in light of
certain limitations. First, this is a secondary data analyses and is
limited in terms of the number of variables or information available in
the data base leading to several unexplained variations in the results.
For example, the lack of key clinical data around weight, height,
hemoglobin and body mass index (BMI) of children, and demographic
information such as parental survival status, economic status limits our
ability to interpret the data. Due to the retrospective nature of this
study, several critical variable were missing for several children whose
data could not be included. Since, the analyses was carried out taking a
cohort of CLHIV who had been initiated on ART over a period six years,
there may have been variations in survival status of children with the
maturity of ART era. Future research could examine the survival status
of children who are on ART at various time points during the ART era and
also understand the reasons behind high mortality among specific
sub-groups of children.
Despite these limitations, the present results
document the mortality rates among a large number of children who have
initiated ART during the early stage of ART introduction in India. The
findings show that children less than five years, those with CD4 count
less than or equal to 200/mm3
at the start of ART, and those in the clinical stage III or IV are more
vulnerable to mortality. Thus, greater emphasis is needed to improve
early HIV diagnosis and treatment in the young age group. Also,
large-scale and long-term research is needed to confirm some of the
current findings and also to ascertain the reasons for high mortality
among children less than five years.
| |
|
What Is Already Known?
•
Previous research has documented a survival probability of
90% in children living with HIV at 12 months.
What This Study Adds?
•
Survival probability of children living with HIV at 12
months in our set-up was 95%.
•
Lower CD4 count, worse clinical staging and functional
status independently determine the mortality among children with
HIV.
|
References
1. National AIDS Control Organisation-National
Institute of Medical Sciences, Technical Report of HIV Estimation. New
Delhi: National AIDS Control Organisation, Ministry of Health and Family
Welfare, Governmnet of India; 2012. p.1-69.
2. National AIDS Control Organisation, Annual Report
2012-13. National AIDS Control Organisation, Ministry of Health and
Family Welfare, Governmnet of India; 2013. p.iv-98
3. Koye DN, Ayele TA, Zeleke BM. Predictors of
mortality among children on antiretroviral therapy at a referral
hospital, Northwest Ethiopia: A retrospective follow up study. BMC
Pediatr. 2012;12:161.
4. Gebremedhin A, Gebremariam S, Haile F,
Weldearegawi B, Decotelli C. Predictors of mortality among HIV-infected
children on anti-retroviral therapy in Mekelle Hospital, Northern
Ethiopia: A retrospective cohort study. BMC Public Health. 2013;13:1047.
5. National AIDS Control Organisation. State Fact
Sheet. New Delhi: Department of AIDS Control, Ministry of Health,
Government of India; 2013. p.7-49
6. Wamalwa DC, Farquhar C, Obimbo EM, Selig S,
Mbori-Ngacha DA, Richardson BA, et al. Early response to highly
active antiretroviral therapy in hiv-1-infected Kenyan children. J
Acquir Immune Defic Syndr. 2007;45:311-7.
7. Wamalwa DC, Obimbo EM, Farquhar C, Richardson BA,
Mbori-Ngacha DA, Inwani I, et al. Predictors of mortality in
HIV-1 infected children on antiretroviral therapy in Kenya: A
prospective cohort. BMC Pediatr. 2010;10:33.
8. Rajasekaran S, Jeyaseelan L, Ravichandran N,
Gomathi C, Thara F, Chandrasekar C. Efficacy of antiretroviral therapy
program in children in India: prognostic factors and survival analysis.
J Trop Pediatr. 2009;55:225-32.
9. Isaakidis P, Raguenaud M-E, Vantha T, S Tray C,
Akao K, Kumar V, et al. High survival and treatment success
sustained after two and three years of first-line ART for children in
Cambodia. J Int AIDS Soc. 2010;13:11.
10. Ryavanki SP KJ, Dayama SO, Mehta A, Solanki N,
Trivedi SS. Survival probabilities of paediatric patients registered in
ART centre at New Civil Hospital, Surat. Natl J Community Med.
2013;4:4-9.
11. Sauvageot D, Schaefer M, Olson D, Pujades-Rodriguez
M, O’Brien DP. Antiretroviral therapy outcomes in resource-limited
settings for HIV-infected children <5 years of Age. Pediatrics. 2010;
125:e1039-47.
12. Callens SF, Kitetele F, Lusiama J, Shabani N,
Edidi S, Colebunders R, et al. Computed CD4 percentage as a
low-cost method for determining pediatric antiretroviral treatment
eligibility. BMC Infect Dis. 2008;8:31.
13. World Health Organization. Consolidated ARV
Guidelines: 2013. Available from:
http://www.who.int/hiv/pub/guidelines/arv2013/art/statartadolescents_rationale/en/.
Accessed March 21, 2016.
|
|
|
 |
|

