|
|
|
Indian Pediatr 2018;55: 292-296 |
 |
Pain Control Interventions in Preterm
Neonates: A Randomized Controlled Trial
|
|
Vivek V Shukla 1,
Satvik Bansal2,
Archana Nimbalkar3,
Apurva Chapla2,
Ajay Phatak4,
Dipen Patel2 and
Somashekhar Nimbalkar2
From 1Division of Neonatology, The Hospital for Sick
Children, Toronto, Canada; Departments of 2Pediatrics and
3Physiology, Pramukhswami Medical College, Karamsad, Anand,
Gujarat, India; and 4Central Research Services, Charutar
Arogya Mandal, Karamsad, Anand, Gujarat, India.
Correspondence to: Dr Vivek Shukla, Division of Neonatology, The
Hospital for Sick Children, 555 University Avenue, Toronto, ON M5G 1X8.
Email: [email protected]
Received: March 31, 2017;
Initial review: June 19, 2017;
Accepted: January 22, 2018.
Published online:
February 09, 2018.
PII:S097475591600114
Trial registration: CTRI/2016/06/007028
|
|
Objectives: To compare individual
efficacy and additive effects of pain control interventions in preterm
neonates.
Design: Randomized controlled
trial
Setting: Level-3 University
affiliated neonatal intensive care unit.
Participants: 200 neonates (26-36
wk gestational age) requiring heel-prick for bedside glucose assessment.
Exclusion criteria were neurologic impairment and critical illness
precluding study interventions.
Intervention: Neonates were
randomly assigned to Kangaroo mother care with Music therapy, Music
therapy, Kangaroo Mother care or Control (no additional intervention)
groups. All groups received expressed breast milk with cup and spoon as
a baseline pain control intervention.
Main outcome measure: Assessment
of pain using Premature Infant Pain Profile (PIPP) score on recorded
videos.
Results: The mean (SD) birth
weight and gestational age of the neonates was 1.9 (0.3) kg and 34 (2.3)
wk, respectively. Analysis of variance showed significant difference in
total PIPP score across groups (P<0.001). Post-hoc comparisons
using Sheffe’s test revealed that the mean (SD) total PIPP score was
significantly lower in Kangaroo mother care group [7.7 (3.9) vs.
11.5 (3.4), 95% CI(–5.9, –1.7), P<0.001] as well as Kangaroo
mother care with Music therapy group [8.5 (3.2) vs. 11.5 (3.4),
95%CI (–5.1, –0.9), P=0.001] as compared to Control group. PIPP
score was not significantly different between Control group and Music
therapy group.
Conclusions: Kangaroo mother care
with and without Music therapy (with expressed breast milk)
significantly reduces pain on heel-prick as compared to expressed breast
milk alone. Kangaroo mother care with expressed breast milk should be
the first choice as a method for pain control in preterm neonates.
Keywords: Kangaroo mother care, Music therapy,
Neonatal pain.
|
|
N
eonates receiving intensive care are subjected to
multiple painful procedures as a part of their intensive care
management. Preterm neonates have immature nociceptive circuitry [1,2].
Pain is linked with abnormal neurodevelopment [3-5], so it is extremely
important to treat and reduce pain. Multiple studies have shown the
benefits of individual pain control interventions [6-11]. There are no
randomized control trials comparing effects of the simultaneous
application of different pain control interventions as compared to their
individual effect on pain.
The objective of this study was to compare the efficacy of two pain
control interventions and interaction effects (if any).
Methods
We conducted the study from January 2016 to May 2016
at a level 3 NICU of Shree Krishna Hospital a University affiliated
teaching hospital, Anand, Gujarat, India. Hospital research ethics
committee approved the trial protocol.
We enrolled preterm neonates (28 to 36 weeks
gestational age) admitted to the NICU after written and informed consent
from their parents. Study interventions were done on babies expected to
have heel-prick procedure for bedside glucose assessment as per the
routine medical management. Exclusion criteria were neurologic
impairment (perinatal depression and HIE
³stage 2 of Sarnat
classification [12], Grade 3/4 IVH [13], stroke, seizures or congenital
malformations of the central nervous system), those who received pain
control medications in 12 hours before study interventions, those with
neonatal abstinence syndrome, and those with critical illness unstable
to undergo study interventions (those requiring mechanical ventilation,
inotropes). The SD of PIPP score [14] was found to be about 3.5 from the
previous study in the same setting [7].
Considering a 2-point difference in PIPP score as
clinically important between any two groups, a sample of size 50 per
group was required at 5% alpha error and 80% power. Considering the
study design, we did not expect dropouts from the study.
Randomization was performed with the use of WINPEPI
software by the statistician and the assignment was placed in sealed
opaque envelopes. The resident involved in the study enrolled the
participants and obtained consent. Neonatologist involved in the study
opened the sealed opaque envelopes and allocated the intervention.
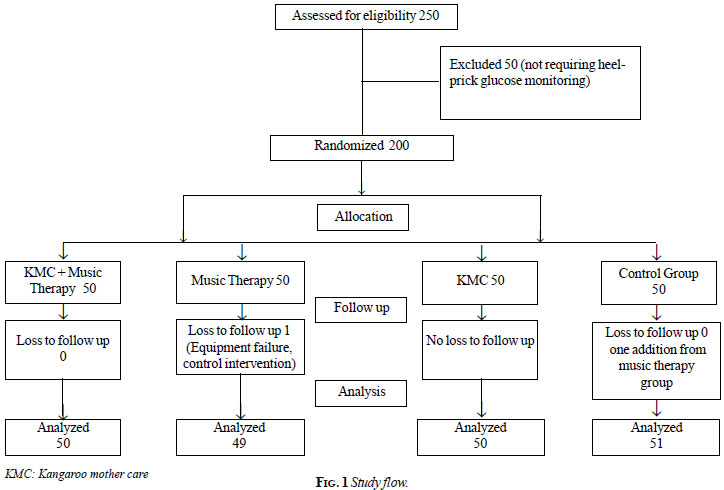
Eligible participants were randomly assigned, in a 1:1 ratio, to
Kangaroo mother care (KMC) with Music therapy (MT) group, Music therapy
group, KMC group or Control (no additional intervention) group (Fig.
1). We provided 2 mL of mother’s expressed breast milk (EBM) with
cup and spoon as baseline pain control measure to all study participants
2 minutes prior to heel-prick in addition to the study interventions.
Study intervention was provided 10 min prior to heel-prick procedure,
and music therapy continued for at least 5 min and KMC was continued
post heel-prick procedure as per institutional protocol. We adhered to
the pain control protocol of the study institute to use EBM for
procedural pain control as a baseline.
 |
| |
In music therapy group, the music was provided from
mobile devices at a distance of 2 feet, and the sound level was between
35 to 45 dBA as measured at the level of ear of the newborn with the
help of Sound Meter PRO (Google Playstore application) from mobile
phone. Efforts were made to minimize noise level in the NICU during
music therapy. The music that was played was instrumental-Indian
classical flute music. In the control group no additional pain control
intervention was provided except for 2 mL of EBM 2 minutes prior to the
heel-prick procedure, they were then swaddled following the video
recording. Video recording of the neonate’s facial expression and pulse-oximetry
monitor which was required for PIPP score calculation was recorded for 5
minutes before and after the intervention. PIPP scoring was done at 30
seconds after the heel-prick procedure. In KMC group infant’s face was
turned to side; care was taken to capture only the facial expression of
the neonate without revealing study interventions, and muted video was
taken for blinded PIPP score assessment. Two fellows in neonatology,
trained in measuring PIPP score, independently assessed the videos for
PIPP score assessment. If the discrepancy was more than 2 points on
total PIPP score, it was resolved through discussion involving the
Neonatologist. Otherwise, the average of the PIPP scores was considered.
Statistical analysis: Analysis of variance
(ANOVA) was employed to compare the total PIPP score across groups.
Scheffe’s test was used for post-hoc comparison to find out the
significant differences between groups. The analysis was performed using
STATA version 14.1.
Results
Parents of all eligible participants were contacted,
and all consented for the study. Fig. 1 provides the study
flow. Out of 200 participants, 104 (52%) were females. The mean (SD)
birthweight and gestational age of the neonates was 1.91 (0.34) kg and
34.0 (2.32) weeks, respectively. The mean (SD) age of the neonates was
8.2 (7.35) days. The baseline characteristics were comparable across
groups (Table I).
TABLE I Baseline Characteristics of Preterm Neonates Receiving Pain-control Interventions
|
Particulars |
KMC (n=50) |
Music Therapy (n=49) |
KMC +Music (n=50) |
Control (n=51) |
Overall (n=200) |
|
Gestational age, wk |
33.9 (2.22) |
33.6 (2.20) |
33.8 (2.87) |
34.6 (1.84) |
34.0 (2.32) |
|
Birthweight, kg
|
1.85 (0.37) |
1.87 (0.32) |
1.94 (0.33) |
1.96 (0.33) |
1.91 (0.34) |
|
Age, d
|
9.0 (8.09) |
8.1 (8.21) |
9.3 (7.94) |
6.5 (4.38) |
8.2 (7.35) |
|
Female gender* |
23 (46) |
23 (46.9) |
27 (54) |
31 (60.8) |
104 (52) |
|
SGA* |
21(42.0) |
19 (38.8) |
28 (56.0) |
23 (45.1) |
91 (45.5) |
|
Values in mean (SD) or *No.(%); SGA: small for gestational
age. |
Analysis of variance revealed that there was a
significant difference in total PIPP score across groups (P<0.001).
A significant difference was also observed in all the individual
components of PIPP score across groups except Behavioral State (P=0.65).
Post-hoc comparisons using Sheffe’s test revealed that the mean (SD)
total PIPP score was significantly lower in KMC group [7.67 (3.93) vs.
11.49 (3.37), 95% CI of difference (–5.90, –1.73), P<0.001] as
well as KMC with Music therapy group [8.50 (3.23) vs. 11.49
(3.37), 95% CI of difference (–5.06, –0.92), P=0.001] as compared
to control group. However, it was similar between control group and
music therapy group (P=0.18). Similar observation was noted for
individual components of PIPP score (Table II).
TABLE II Comparison of PIPP Scores Across Groups (N=200)
|
PIPP Components |
KMC |
Music Therapy |
KMC +Music |
Control |
Overall |
|
(n= 50)
|
(n=49) |
(n=50) |
(n=51) |
(n=200) |
|
Gestational age
|
0.8 (0.8) |
0.9 (0.7) |
0.8 (0.8) |
0.5 (0.6) |
0.8 (0.8) |
|
Behavioral state
|
2.5 (0.9) |
2.4 (0.9) |
2.3 (1.1) |
2.3 (0.9) |
2.3 (0.9) |
|
Heart rate
|
0.9 (0.8) |
1.1 (0.7) |
0.9 (0.7) |
1.5 (0.7) |
1.1 (0.77) |
|
Oxygen saturation (SpO2)
|
0.3 (0) |
0.6 (0.6) |
0.4 (0.6) |
0.8 (0.7) |
0.57 (0.66) |
|
Brow bulge
|
1.2 (1.2) |
1.8 (1.1) |
1.6 (1.1) |
2.3 (0.9) |
1.72 (1.15) |
|
Eye squeeze
|
1.1 (1.2) |
1.6 (1.1) |
1.3 (1.0) |
2.2 (1.1) |
1.55 (1.15) |
|
Naso-labial furrow
|
0.8 (1.0) |
1.4 (1.1) |
1.1 (1.0) |
1.9 (1.1) |
1.32 (1.14) |
|
Total PIPP score
|
7.7 (3.9) |
9.9 (4.2) |
8.5 (3.2) |
11.5 (3.4) |
9.40 (3.95) |
|
PIPP: Premature infant pain profile; All values in mean
(SD). |
No study intervention related side effects were
encountered in any participants.
Discussion
Pain research in preterms, till date, has been
focused on individual pain control interventions (Kangaroo mother care
[6,7,15-18], music therapy [8,9,19,20] and expressed breast milk
[10,11,21]) but they have not been studied for comparison and additive
effects. The results of present study revealed that pain control
interventions have different efficacy individually and when combined on
total PIPP score as well as on majority of its individual components.
KMC with EBM was found to be the most efficacious method in reducing
neonatal pain.
Flute-based music therapy was not shown to have
additive benefit when combined with KMC and EBM. It is however possible
that different kind of music may give different results albeit it is
difficult to test it with current research design that contained only
one type of music. We chose flute-based music as it has been shown to
have pain-modifying effect in adults [22,23]. Because of ethical
considerations expressed breast milk was provided as baseline for all
study participants. Due to this baseline intervention, individual
efficacy of study interventions might have been impacted. We included
neonates born at 28 to 36 weeks of gestational age for increasing the
generalizability of the study results across extended gestational age
group; however, the individual efficacy of interventions in particular
gestational ages may be different and was not studied, as that was not
the focus of present research.
Sucrose, although routinely used for pain control in
neonates, has not been adequately studied for long-term side effects.
Concerns have been raised about neurodevelopmental effects of sucrose
when used in preterm babies in multiple doses [24].
Given that preterm neonates receive multiple
painful procedures, it is imperative to use non-pharmacological methods
like Kangaroo mother care, music therapy and expressed breast milk.
KMC and KMC with music therapy (with EBM for baseline
pain control) significantly reduces pain on heel-stick as compared to
control (EBM alone). Increased efficacy of KMC and EBM should be
investigated further in different study settings to enhance
generalizability and should be practiced in current study settings
considering KMC and EBM provides additional benefits of promoting
breastfeeding and mother infant bonding. KMC with EBM should be the
first choice as a method for pain control in preterm neonates. Further
studies comparing pain control interventions for confirming the present
findings and to assess long-term neuro-developmental outcome
implications with better pain control are warranted.
Contributors: VS: conceptualized and planned the
study, drafted the proposal and manuscript, and supervised data
collection; SB: planned the study design, and revised the manuscript for
important intellectual points; AN: conceptualized and devised the study,
analyzed the data, and contributed to manuscript writing; AC:
contributed to data collection, analysis of study, and drafting the
manuscript;; AP: contributed to study design, analyzed the data,
provided important intellectual inputs to the manuscript; DP:
contributed to study design, supervised the study, and contributed to
manuscript writing; SN: conceptualized, planned and supervised the
progress of the study, analyzed the data, and provided important
intellectual inputs to the manuscript. All authors approved the final
version of manuscript.
Funding: None; Competing interests: None
stated.
|
What is Already Known?
•
Kangaroo mother care, music therapy and expressed breast
milk are individually effective interventions for pain control
in preterm neonates.
What This Study Adds?
• Kangaroo mother care
has an additive effect when combined with expressed breast milk
or with expressed breast milk and music therapy, showing
significantly better pain control as compared to expressed
breast milk alone.
|
References
1. Fitzgerald M. The development of nociceptive
circuits. Nat Rev Neurosci. 2005;6:507-20.
2. Fitzgerald M, Walker SM. Infant pain management: a
developmental neurobiological approach. Nat Clin Pract Neurol.
2009;5:35-50.
3. Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant
R, Vinall J, et al. Procedural pain and brain development in
premature newborns. Ann Neurol. 2012;71:385-96.
4. Ranger M, Chau CM, Garg A, Woodward TS, Beg MF,
Bjornson B, et al. Neonatal pain-related stress predicts cortical
thickness at age 7 years in children born very preterm. PloS One.
2013;8:e76702.
5. Valeri BO, Holsti L, Linhares MB. Neonatal pain
and developmental outcomes in children born preterm: a systematic
review. Clin J Pain. 2015;31:355-62.
6. Lyngstad LT, Tandberg BS, Storm H, Ekeberg BL,
Moen A. Does skin-to-skin contact reduce stress during diaper change in
preterm infants? Early Hum Dev. 2014;90: 169-72.
7. Nimbalkar SM, Chaudhary NS, Gadhavi KV, Phatak A.
Kangaroo mother care in reducing pain in preterm neonates on heel prick.
Indian J Pediatr. 2013;80:6-10.
8. Bergomi P, Chieppi M, Maini A, Mugnos T, Spotti D,
Tzialla C, et al. Nonpharmacological techniques to reduce pain in
preterm infants who receive heel-lance procedure: a randomized
controlled trial. Res Theory Nurs Pract. 2014;28:335-48.
9. Thiel MT, Findeisen B, Langler A. Music therapy as
part of integrative neonatology: 20 years of experience - 3 case reports
and a review. Forsch Komplementmed. 2011;18:31-5.
10. Simonse E, Mulder PG, van Beek RH. Analgesic
effect of breast milk versus sucrose for analgesia during heel lance in
late preterm infants. Pediatrics. 2012;129:657-63.
11. Codipietro L, Ceccarelli M, Ponzone A.
Breastfeeding or oral sucrose solution in term neonates receiving heel
lance: a randomized, controlled trial. Pediatrics. 2008;122: e716-21.
12. Sarnat HB, Sarnat MS. Neonatal encephalopathy
following fetal distress. A clinical and electroencephalographic study.
ArchNeurol. 1976;33:696-705.
13. Papile LA, Munsick-Bruno G, Schaefer A.
Relationship of cerebral intraventricular hemorrhage and early childhood
neurologic handicaps. J Pediatr. 1983;103:273-7.
14. Stevens B, Johnston C, Petryshen P, Taddio A.
Premature Infant Pain Profile: development and initial validation. Clin
J Pain. 1996;12:13-22.
15. Zwicker JG, Grunau RE, Adams E, Chau V, Brant R,
Poskitt KJ, et al. Score for neonatal acute physiology-II and
neonatal pain predict corticospinal tract development in premature
newborns. Pediatr Neurol. 2013;48:123-9.e1.
16. Campbell-Yeo M, Johnston C, Benoit B, Latimer M,
Vincer M, Walker CD, et al. Trial of repeated analgesia with
Kangaroo mother care (TRAKC Trial). BMC Pediatr. 2013;13:182.
17. Johnston C, Campbell-Yeo M, Fernandes A, Inglis
D, Streiner D, Zee R. Skin-to-skin care for procedural pain in neonates.
Cochrane Database Syst Rev. 2014;1:CD008435.
18. Mitchell AJ, Yates CC, Williams DK, Chang JY,
Hall RW. Does daily kangaroo care provide sustained pain and stress
relief in preterm infants? J Neonat Perinat Med. 2013;6: 45-52.
19. Wright J, Adams D, Vohra S. Complementary,
holistic, and integrative medicine: music for procedural pain. Pediatr
Rev. 2013;34:e42-6.
20. Butt ML, Kisilevsky BS. Music modulates behaviour
of premature infants following heel lance. Can J Nurs Res.
2000;31:17-39.
21. Sahebihag MH, Hosseinzadeh M, Mohammadpourasl A,
Kosha A. The effect of breastfeeding, oral sucrose and combination of
oral sucrose and breastfeeding in infant’s pain relief during the
vaccination. Iran J Nurs Midwifery Res. 2010;16:1-7.
22. Ikonomidou E, Rehnstrom A, Naesh O. Effect of
music on vital signs and postoperative pain. AORN J. 2004;80:269-74,
77-8.
23. McCaffrey R, Freeman E. Effect of music on
chronic osteoarthritis pain in older people. J Adv Nurs. 2003;44:517-24.
24. Johnston CC, Filion F, Snider L, Majnemer A,
Limperopoulos C, Walker CD, et al. Routine sucrose analgesia
during the first week of life in neonates younger than 31 weeks’
postconceptional age. Pediatrics. 2002;110:523-8.
|
|
|
 |
|

