|
|
|
Indian Pediatr 2017;54:
279-283 |
 |
Comparative Efficacy and Safety of Caffeine
and Aminophylline for Apnea of Prematurity in Preterm ( £34
weeks) Neonates: A Randomized Controlled Trial
|
|
M Shivakumar, P Jayashree, Muhammad Najih, Leslie
Edward Simon Lewis,
Ramesh Bhat Y,
*Asha Kamath and Shashikala
From Departments of Pediatrics and *Community
Medicine, Women and Child block, Kasturba Hospital, Manipal.
Correspondence to: Dr Leslie Edward Simon Lewis,
Professor and Unit Head, Neonatal Intensive Care Unit,
Department of Pediatrics, Kasturba Hospital, Manipal University Manipal
576 104, India.
Email: [email protected]
Received: May 21, 2016;
Initial Review: August 31, 2016;
Accepted: February 12, 2017.
Trial Registration: CTRI/2012/08/002904
|
Objective: To compare the
efficacy and safety of standard doses of Caffeine and Aminophylline for
Apnea of prematurity.
Study design: Randomized
controlled trial.
Setting: Tertiary-care referral
centre and a teaching institution in Southern India. Trial was conducted
from February 2012 to January 2015.
Participants: 240 preterm ( £34
wk) neonates with apnea of prematurity.
Interventions: Neonates
randomized into two groups: Caffeine group received loading dose of
caffeine citrate (20 mg/kg) followed by 5 mg/kg/day maintenance dose
every 24 hour. Aminophylline group received loading dose of
Aminophylline – 5 mg/kg and maintenance dose of 1.5 mg/kg 8-hourly.
Outcome measures: Difference in
apneic spells, associated respiratory morbidity, and acute adverse
events were assessed. Association of efficacy with therapeutic drug
levels was also evaluated.
Results: Infants on aminophylline
experienced less apnea spells in 4-7 days of therapy (P=0.03).
Mean apnea rate and isolated desaturations were similar in 1-3, 4-7 and
8-14 days of therapy. No difference was noted in duration of Neonatal
Intensive Care Unit stay and hospital stay. Mean heart rate was
significantly high in Aminophylline group (P<0.001). Risk of
developing tachycardia was less (RR 0.30; 95% CI range 0.15 to 0.60;
P<0.001) in Caffeine- over Aminophylline-treated infants.
Conclusion: Aminophylline is as
effective as caffeine for prevention of apneic spells in preterm
neonates; however, dosage optimization needs to be done to reduce
toxicity.
Keywords: Apneic spells, Methylxanthines,
Preterm neonates.
|
|
A
pnea of prematurity (AOP) itself
is not a major threat to infant health but frequent recurrent episodes
accompanied by hypoxemia and bradycardia significantly causes brain
damage in preterm population [1]. A cochrane meta-analysis has reported
significant reduction in apneic episodes and subsequent usage of
mechanical ventilation in neonates treated with methylxanthines [2].
There are limited trials emphasizing effectiveness
and safety on caffeine versus aminophylline in developing
country. Besides, Small for Gestation Age (SGA) growth category or
Intrauterine Growth Retarded (IUGR) babies are a significant problem in
many developing and underdeveloped countries, and the effect of
methylxanthines in them is incompletely understood [3]. Therefore the
present study was designed to compare the effectiveness of
methylxanthines in preterms, particularly the SGA babies.
Methods
This single centered, parallel, open label,
randomized controlled trial was conducted from February 2012 to January
2015 in a tertiary level Neonatal Intensive Care Unit (NICU) at Kasturba
Hospital, Manipal University. The study was approved by the
Institutional Ethics committee, and written informed parental consent
was obtained for each participating infant.
Preterm newborns with
£34
completed weeks of GA who experienced six or more apneic spells in 24
hours, or preterm neonates with apneic episode requiring bag and mask
ventilation for termination of apnea were included. Sepsis work-up,
echocardiography, relevant blood, and radiological investigations were
done at inclusion in order to evaluate and exclude neonates with
secondary causes of apnea. Investigations were further repeated based on
clinical signs. Some other exclusion factors included major congenital
anomalies, respiratory depression from medications and Patent Ductus
Arteriosus (PDA) as a cause of apnea (defined as - ductus diameter of
1.5 mm and absent/ retrograde diastolic flow in the post-ductal aorta).
Computer generated block randomization was used with
block size of 10. Allocation concealment was executed by using
sequentially numbered, sealed, opaque envelops. Both random allocation
sequencing and concealment was done by research officer who was not
concerned with current trial or in management of recruited infants. The
treatment assignment was carried out by attending clinician in
Neonatology unit.
Infants allocated to Caffeine group received a
loading dose of 20 mg/kg of caffeine citrate (10 mg/kg caffeine base)
diluted in 5% dextrose given for 30 minutes and were continued on a
maintenance dose of 5 mg/kg (2.5 mg caffeine base) 24 hourly (iv or oral
preparation of caffeine citrate solution 20 mg/mL). If adequate response
was not seen then the dose was optimized up to 7.5 mg/kg.
Neonates allocated to aminophylline group received a loading dose of 5
mg/kg of aminophylline, diluted in 5 % dextrose followed with a
maintenance dose of 1.5 mg/kg 8 hourly (Inj. aminophylline 250 mg/10 mL).
If adequate response was not seen, the dose was titrated up to 2 mg/kg.
Baseline parameters were measured for each
participant at inclusion. Gestational age was calculated from maternal
menstrual history or from first trimester ultrasound scan. If neither of
these were available or in case of discrepancies, a new Ballard’s
assessment was performed and considered as final. Intrauterine Growth
categorization was done at birth based on Lubchenco growth chart. The
data on antenatal steroids, APGAR score at 1 and 5 minutes, need for
surfactant, gender and birth weight was noted. Continuous monitoring of
vitals and SpO 2
was done by Phillips IntelliVue MP20 neonatal monitors with alarms set
to alert at SpO2
<85% saturation and heart rate (HR) <100 bpm; SpO2
of 90-95% was targeted. Clinical assessment was done every 24 hours
after commencement of methylxanthines. Daily apneic episodes, isolated
desaturations, intervention used, mean of 24 hours HR and adverse
effects were recorded. Neonates were discharged when 1800 g in weight,
self-feeding, euthermic, and apnea-free for 7 days off- methylxanthines.
Follow-up of these high risk neonates was done as per unit protocol.
After reaching plasma concentration (4-5 t 1/2
after initiation of therapy) a
blood sample (0.25 mL) was taken to measure plasma theophylline and
caffeine levels aiming to achieve a therapeutic concentration (5 to 12
mg/L theophylline and 5 to 20 mg/L caffeine). Sampling was done at the
trough levels before the next dose was due. Sampling was planned during
change in IV lines or with other blood samples to avoid unnecessary
sampling pricks. Plasma caffeine and theophylline concentrations were
quantified with LCMS assay.
Primary outcome was frequency of apneic episodes
(number of apnea spells per 24 hour) at an interval of 1 to 3 day, 4 to
7 day and 8 to 14 day of therapy. Secondary outcomes were Mean apnea
rate (MAR) (defined as the average number of desaturations with
bradycardia per neonate over 24 hours period), frequency of
desaturations (Number of isolated desaturation per 24 hour) recorded in
set interval of 1 to 3 day, 4 to 7 day and 8 to 14 day of therapy, time
for apnea resolution, duration of hospital stay, HR variability on 1 st,
2nd, 3rd,
7th and 14th
day of methylxanthine therapy, and safety profile with respect to some
known reported adverse events of methylxanthines (tachycardia,
jitteriness, feed intolerance and abnormal blood sugar).
Sample size: Based on anticipated SD of 5 apneic
episodes in 24 hours and minimal relevant difference at 3 continuing
apneic episodes in 24 hours, a power of 90%, 5% as a level of
significance, it was estimated that 84 neonates would be needed in each
arm. Assuming 30% attrition rate, a sample of 120 in each arm was taken.
Statistical analysis: Mann- Whitney U test was
used to compare episodes of apnea, MAR, isolated desaturations, time
taken for apnea resolution, NICU stay and hospital stay. Repeated
measures ANOVA was used to compare changes in HR at various time
periods. Difference in Mean HR on individual day was analyzed by
applying Independent sample t test. Adverse effects and
co-morbidities were analyzed by Pearson’s chi-square test. Association
of occurrence of apnea and isolated desaturation was analyzed by
Pearson’s chi-square test. A subgroup analysis for the outcomes was
carried out stratified by intrauterine growth status. In each growth
strata the difference in apnea spells, MAR, desaturation frequencies and
adverse events between the groups were compared using generalized linear
model with poisson loglinear scale response. Factorial ANOVA was adopted
to compare significant difference in Median days of apnea resolution,
hospital stay, NICU stay, and Mean HR between groups in different growth
category. P<0.05 was considered to be statistically significant.
A Data Safety Management Board (DSMB) periodically
reviewed the study progress.
Results
During the period of 36 months, out of admitted
preterm neonates, 240 (23%) newborns were identified experiencing AOP
and underwent randomization. The study flow is depicted in Fig.
1. Baseline characteristics were similar in both the groups (Table
I).
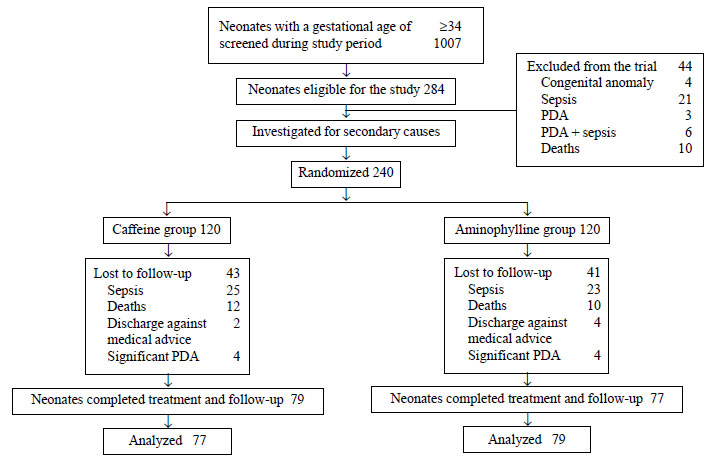 |
|
Fig.1 Study flow.
|
TABLE I Baseline Demographics Characteristics
Characteristic
|
Caffeine
(n=120)(%) |
Aminophylline
(n=120)(%) |
|
Born at study hospital |
100 (83.3) |
104 (86.7) |
|
Antenatal steroids |
|
Complete |
27 (22.5) |
32 (26.7) |
|
Partial |
27 (22.5) |
20 (16.7) |
|
SGA |
27 (22.5) |
28 (23.3) |
|
Delivery by Caesarean section |
87 (72.5) |
77 (64.2) |
|
Low APGAR at 1min (<7) |
59 (49.2) |
51 (42.5) |
|
Low APGAR at 5min (<7) |
22 (18.3) |
19 (15.8) |
|
Female sex |
56 (46.7) |
66 (55) |
|
Surfactant received |
68 (56.7) |
57 (47.5) |
|
Birth weight (g) (mean, SD) |
1149.7 (307) |
1155.9 (313) |
|
Gestational age, wks (mean, SD) |
29.4 (2) |
29.3 (1.9) |
TABLE II Comparison of Apnea Frequency and Secondary Outcomes
|
Variable |
Caffeine |
Aminophylline |
P |
|
(n=77) |
(n=79) |
value |
|
Continuing apnea at days of therapy* |
|
1-3 d |
0 (0,14) |
0 (0,5) |
0.03 |
|
4-7 d |
0 (0,20) |
0 (0,8) |
0.05 |
|
8-14 d |
0 (0,15) |
0 (0,13) |
0.82 |
|
Apnea rate (per 24hrs) at days of therapy* |
|
1-3 d |
0 (0,7) |
0 (0, 1) |
0.53 |
|
4-7 d |
0 (0,9) |
0 (0,4) |
0.27 |
|
8-14 d |
0 (0,5) |
0 (0,2) |
0.12 |
|
Isolated desaturations (per 24hrs) at days of therapy* |
|
1-3 d |
0 (0,13) |
0 (0,8) |
0.12 |
|
4-7 d |
0 (0,15) |
0 (0,25) |
0.24 |
|
8-14 d |
0 (0,20) |
0 (0,90) |
0.50 |
|
Time taken for |
6 (1, 19) |
6 (1, 16) |
0.89 |
|
apnea resolution (d)$ |
|
NICU stay (d)$ |
38 (23, 55.5) |
35 (24, 48) |
0.45 |
|
Hospital stay (d)$ |
43 (27.5, 61.5) |
39 (28, 55) |
0.43 |
|
Heart rate trend (beats per min)‡ |
|
Day 1 |
143.4 (10) |
143.4 (9.2) |
<0.001 |
|
Day 2 |
144.9 (10.4) |
147.9 (10.2) |
|
|
Day 3 |
143.8 (10.5) |
148.8 (11.4) |
|
|
Day 7 |
149.3 (11.2) |
150.7 (11.3) |
|
|
Day 14 |
147.4 (8.7) |
147.4 (9.9) |
|
|
*values representing in Median (minimum, maximum); $Median
(IQR); ‡Mean(SD). |
Apneic episodes during 4-7 days of therapy was found
to be significantly higher in Caffeine group (P=0.03). Complete
resolution of apnea was achieved after similar duration of median 6 days
in either group. There was no difference in median (IQR) duration of
methylxanthine administration [24 (14, 35.5) d vs 22 (14, 38) d;
P=0.97]. No difference was noted in median length of hospital
stay and NICU stay between both the groups. Mean HR was significantly
higher in aminophylline group at various time intervals (P=0.001)
(Table II). Among caffeine-treated neonates, the risk of
developing tachycardia was lesser than Aminophylline group (RR 0.30; 95%
CI range 0.15 to 0.60, P<0.001). No significant difference found
in the risk of developing feed intolerance (17% vs 22.8%, RR
0.74; 95% CI range 0.39 to 1.40, P=0.35). jitteriness (8% vs
9%, RR 0.87; 95% CI range 0.31 to 2.49, P=0.81) and glucose
abnormality (3% vs 3%, RR 1.02; 95% CI range 0.21 to 4.92, P=0.97)
were also similar in both the groups.
In 42 neonates who were on standard maintenance doses
of caffeine, mean (SD) plasma caffeine level was 14.4 (5) mg/L.
Approximately 93% (n=39) fell within a recommended wider
therapeutic range whereas only 7.1% (n=3) had exceeded
therapeutic window. Odds ratio (95% CI) of occurrence of apnea and
isolated desaturation in the recommended therapeutic range of caffeine
was 0.34 (0.02, 6.04) and 0.09 (0.007, 1.17), respectively as that of
supra-therapeutic levels. In 50 neonates on aminophylline, median (IQR)
level of the drug was 12.9 (6.3, 19.5) from minimum 0.68 to maximum
50.37 mg/L. Majority of them 52% (n=26) had achieved above the
therapeutic range. 24% of neonates had attained therapeutic (n=12)
and sub-therapeutic range (n=12). Odds (95% CI) of occurrence of
apnea and isolated desaturation in the recommended therapeutic range of
aminophylline was 0.30 (0.05, 1.58), and 0.93 (0.08, 10.0) as that of
inadequate therapeutic levels, respectively.
One neonate was LGA from each group were excluded for
subgroup analysis as the number of LGA babies were too less to analyze.
In AGA category continuing apnea episodes during first week of therapy
was consistently high in Caffeine group [1-3 days (P=0.01) and
4-7 days (P<0.001)]. During 4-7 days of therapy caffeine group
proved to have higher MAR (P=0.001). Persistent desaturation in
caffeine group was more than aminophylline during first 3 days of
therapy (P=0.006) than in second week of therapy, aminophylline
group reported high desaturation episodes (P=<0.001). Among SGA
infants, during first 3 days of therapy apnea episodes were higher in
caffeine group (P=0.01). Neonates in caffeine group had
significant higher isolated desaturations during first week of treatment
[1-3 days (P<0.001) and 4-7 days (P<0.001)]. In AGA
babies, aminophylline group had higher mean HR on day 2 (P=0.007)
and day 3 (P=0.002) (Table III).
TABLE III Comparison of Apnea in AGA and SGA Neonates Treated by Caffeine and Aminophylline
|
Appropriate for Gestational Age infants |
Small for Gestational Age infants |
|
C(n=57) |
A(n=58) |
RR (95% CI) |
C(n=19) |
A(n=20) |
RR (95% CI) |
|
Apnea* (Number of episodes per 24hrs) |
|
1-3 days |
2.1 (2.1) |
1.5 (1.7) |
1.9 (1.2 to 3.0) |
2.1 (1.7) |
1.8 (1.9) |
2.5 (1.2 to 5.0) |
|
4-7 days |
2.1 (2.6) |
1.9 (1.9) |
2.3 (1.5 to 3.5) |
1.8 (1.5) |
4.9 (2) |
1.4 (0.7 to 3.1) |
|
8-14 days |
3.3 (2.7) |
2.3 (2.2) |
1.3 (0.9 to 1.8) |
3.3 (1.7) |
5.9 (2.3) |
0.8 (0.5 to 1.3) |
|
Apnea Rate* |
|
1-3 days |
3.7 (2.4) |
0 |
9.2 (1.2 to 72.2) |
0 |
0 |
- |
|
4- 7 days |
3.6 (2.1) |
1.9 (1.9) |
3.9 (1.7 to 9.0) |
0 |
0 |
- |
|
8-14 days |
2.8 (1.8) |
0 |
16.2 (2.1 to 122.7) |
0 |
0 |
- |
|
Desaturation* (Number of episodes per 24hrs) |
|
1-3 days |
3.6 (2.4) |
2.1 (2) |
1.8 (1.2 to 2.8) |
4 (2) |
1 (1) |
20.5 (4.9 to 85.0) |
|
4-7 days |
4.8 (2.6) |
4 (3) |
1.1 (0.8 to 1.5) |
4.6 (1.8) |
4 (0) |
6.8 (2.4 to 19.6) |
|
8-14 days |
4.2 (2.5) |
5.9 (3.4) |
0.5 (0.4 to 0.7) |
2.3 (1.9) |
1.9 (2.5) |
1.2 (0.6 to 2.5) |
|
Heart rate trend (beats per min)# $ |
|
Day 1 |
142.8 (9.5) |
143.3 (8.2) |
0.75 |
145.4 (11.5) |
142.5 (10.6) |
0.34 |
|
Day 2 |
143.6 (10.1) |
148.8 (9.7) |
0.007 |
147.6 (9.7) |
144.9 (11.1) |
0.40 |
|
Day 3 |
145.3 (10.1) |
149.6 (11.4) |
0.002 |
145.7 (11.7) |
146.4 (11.5) |
0.86 |
|
Day 7 |
149.7 (12.0) |
151.2 (11.5) |
0.47 |
148.6 (8.6) |
148.3 (10.6) |
0.93 |
|
Day 14 |
146.9 (8.2) |
147.6 (10.4) |
0.69 |
149.4 (9.8) |
146.3 (8.2) |
0.30 |
|
*Exponential values for Mean (SD) of logarithmic form;
#Data is presented in Mean(SD);
$Comparison done by two-way ANOVA and P
value is presented. |
Discussion
In present study, apneic episodes were found to be
similar in both the caffeine- and aminophylline-treated groups during
first 3 days and second week of therapy. No differences were also noted
in MAR, isolated desaturations and time to resolution of apnea. Risk of
developing tachycardia in caffeine treated neonates was less compared to
aminophylline group. However, there were significant differences noted
in efficacy or adverse events between caffeine and aminophylline in
subgroup of AGA and SGA neonates.
Limitations of present study was that it was a single
center, open label study. Attending clinicians were not blinded. There
are only few trials reported that have compared the effectiveness and
safety of caffeine and aminophylline [4-7]. Most of the trials were
conducted in developed countries. A recent study [8] compared the
incidence of apnea between caffeine and aminophylline treated babies and
followed up recruited neonates for 10 days of life. The study reported
infants with aminophylline dihydrate had approximately 10% less risk of
developing apnea compared to anhydrous caffeine. The results of the
present study were comparable to that of Scanlon, et al. [6] that
standard doses of aminophylline resulted in less apnea frequency in
first week of therapy when compared with standard doses of caffeine.
Tachycardia was the major acute adverse event noted in aminophylline-treated
neonates and was comparable to observation with other studies [4-8].
In conclusion both caffeine and aminophylline are
equally effective in reducing apneas. Perhaps further research should be
undertaken to compare the safety and effectiveness of methylxanthines in
AGA and SGA separately. Since tachycardia is prominently seen in
aminophylline-treated infants, dosage optimization of aminophylline can
be tried for preterm population in developing countries. Aminophylline
usage can be continued under close supervision at resource poor settings
in India.
Contributors: SM, MN, Shashikala:
acquisition of data, drafting the article or analysis and interpretation
of data; LEL, JP, RBY: substantial contribution to concept and design,
revising the work critically for important intellectual content and
final approval of the version published; AK: revising the work
critically on research methodology and Statistical guidance: SM:
co-author who should be approached for the access to raw data.
Funding: Indian Council of Medical Research, New
Delhi.
Competing interest: None stated.
|
What is Already Known?
• Both caffeine and aminophylline are
used for reducing apneic spells in preterm babies with apnea of
prematurity.
What This Study Adds?
•
Aminophylline administration is as effective as caffeine for
apnea of prematurity both in AGA and SGA category, and can
continued to be used in resource-poor setting.
|
References
1. Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s
new? Pediatr Pulmonol. 2008;43:937-44.
2. Henderson-Smart DJ, De Paoli AG. Methylxanthine
treatment for apnoea in preterm infants. Cochrane Database Syst Rev.
2010;12: CD000432.
3. Chaudhuri M, Garg SK, Narang A, Bhakoo ON.
Kinetics of theophylline in apnea of prematurity in small for
gestational age babies. Indian Pediatr. 1996;33:181-7.
4. Larsen PB, Brendstrup L, Skov L, Flachs H.
Aminophylline versus caffeine citrate for apnea and bradycardia
prophylaxis in premature neonates. Acta Paediatr Oslo Nor 1992.
1995;84:360-4.
5. Brouard C, Moriette G, Murat I, Flouvat B, Pajot
N, Walti H, et al. Comparative efficacy of theophylline and
caffeine in the treatment of idiopathic apnea in premature infants. Am J
Dis Child 1960. 1985;139:698-700.
6. Scanlon JE, Chin KC, Morgan ME, Durbin GM, Hale
KA, Brown SS. Caffeine or theophylline for neonatal apnoea? Arch Dis
Child. 1992;67:425-8.
7. Hendy H, Wandita S, Kardana IM. Efficacy of
aminophylline vs caffeine for preventing apnea of prematurity.
Paediatrica Indonesiana. 2014;54:365-71.
8. Skouroliakou M, Bacopoulou F, Markantonis SL.
Caffeine versus theophylline for apnea of prematurity: a randomised
controlled trial. J Paediatr Child Health. 2009;45:587-92.
|
|
|
 |
|

