The section on Methods is the most vital part of a
study as well as of a manuscript. It is the most critically evaluated
section of a paper not only by the reviewers but often by the readers as
well. It should always be written in a simple and clearly understandable
language, and should be objective. Apart from being the most vital part,
it is also generally the lengthiest section of any research paper,
unless a methodology paper for that research is published separately
[1]. While writing this section, the authors must ensure that it is
crisp, concise and complete in every aspect to address all the queries
pertaining to the study like ‘who’, ‘where’, ‘when’, ‘what’ and ‘how’
[2].
General Concepts, Framework and Writing Style
This section, while being written for a research
paper, must present exactly the same information as in ‘Material and
Methods’ section of the study protocol or thesis. Thus, it must be
meticulously built up, giving the maximum time and thought, while
preparing the research protocol. A precise and objectively written
methods section in a protocol will save time and efforts, as often it
can be simply replicated in the final manuscript. The main difference
between the two would be of the tense i.e. future tense in the
protocol and past tense in the paper [3]. Often, authors need to
truncate the ‘Methods’ in the final paper so as to match with the
Results that are presented for that particular paper, and also to avoid
unnecessary details keeping in mind the recommended word count limits of
the journal. Describe methodology in such a manner that it has all the
information (including references) reader needs to replicate the
study/experiment without access to the detailed study protocol [4].
The authors must ensure fluency while writing the
methods. A common mistake that makes the write-up appear disjointed is
use of both passive as well as active voice within the same section. For
example,
"The participants were recruited from the outpatient
department of the xyz hospital. We collected blood samples from all the
patients. The samples were stored at -80 °C. We analyzed the
serum antibody levels of the stored samples after all the patients were
recruited."
One must stick to either the point of view of the
experiment (passive voice) or the point of view of the experimenter
(active voice) throughout a paragraph to ensure coherence [5]. Though
style manuals prefer the active voice for medical and scientific
writing, the passive voice has its place in the Methods section. Hence,
you can alternatively modify the above statement in either of the
following ways:
"We recruited participants from the outpatient
department of the xyz hospital. We collected blood samples from all the
patients, and stored the samples at –80 °C. We analyzed the serum
antibody levels of the stored samples after recruiting all the patients"
Or
"The participants were recruited from the outpatient
department of the xyz hospital. Blood samples from all the patients were
collected, and stored at –80 °C. The serum antibody levels of the
stored samples were analyzed after all the patients were recruited."
You can present the methodology in a structured or an
unstructured format, depending on the journal’s requirements. Structured
representation makes it more clear and objective for easier
comparability between different studies. Unstructured format, though
lacks objectivity, may be a better option for some types of studies like
descriptive and qualitative studies [6]. Print journals often prefer
unstructured format to save space. Irrespective of the framework, the
components of this section essentially remain the same, and have been
provided in Box 1 as a checklist.
|
Box 1 Checklist for Methods Section |
|
• Study setting and place
• Duration
• Study design
• Ethical considerations
• Consent and assent
• Funding information
• Patient confidentiality
• Trial registration details
(if any)
• Follow standard reporting
guidelines
• Participant selection and
sampling
Definition of participants
(cases and controls)
Sampling technique used
Inclusion criteria
Exclusion criteria
• Method of randomization
(for controlled studies), including group allocation details
• Blinding details (if
applicable)
• Exact
procedure/Intervention (with references)
• Primary and secondary
outcome variables
• Sample size calculation
• Statistical analysis
|
Study Setting, Duration and Design
Study setting description involves mentioning the
place where the study was conducted which may be more than one in case
of multi-centric studies. Specify the place from where the
patients/samples/clinical records were recruited/obtained like
out-patient department/inpatient department/emergency/medical record
department, for a hospital-based study. The place of study may be a
school, village or district in case of a community-based study. One
should also mention all the departments involved in carrying out the
study apart from the primary department. The exact duration over which
the study was carried out must also be specified, including period of
enrolment of participants and their follow-up.
Study design must be specified in the beginning of
the Methods section. Medical research is broadly classified as primary
and secondary. Secondary research involves summarizing the results
available from primary research in the form of systematic reviews and
meta-analyses. Primary research that involves synthesizing the evidence
can broadly be classified into basic medical research, clinical research
and epidemiological research; though there is no rigid demarcation in
these study areas [7]. The commonly used epidemiological study designs
are depicted in Fig. 1. Descriptive studies help to
generate a hypothesis by simple description of certain population
parameters and finding some associations. Analytical studies help test
such hypothesis to establish causation. Analytical studies can be
observational or experimental. The purpose of an observational study is
to follow the natural course of events in one or more groups formed on
the basis of presence/absence of exposure, risk factor or disease.
Whereas in an experimental study, the investigator intentionally
manipulates one or more independent variables after controlling the
effect of potential confounders and analyzes the results of that
intervention [8]. Apart from these, there are certain areas of special
research like qualitative research, decision analysis, operations
research, health systems research, quality assurance,
cost-effectiveness/ economic analysis, which are beyond the scope of
this article.
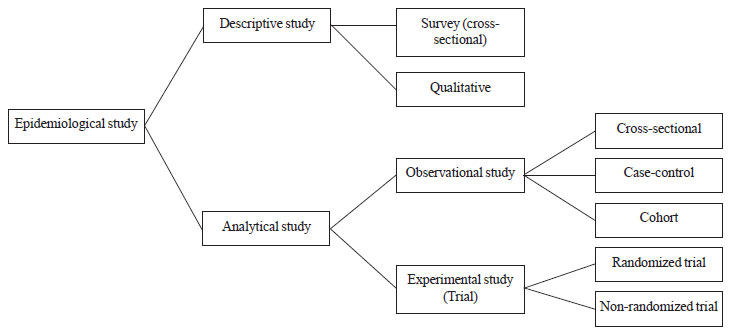 |
|
Fig. 1: Some common epidemiological
study designs.
|
The exact layout of Methods section depends on the
type of study design. Nowadays, majority of the journals recommend the
authors to adhere to the respective reporting guidelines for different
types of studies to promote transparent, accurate and good quality
reporting (Table I) [4,9-17]. These guidelines help the
authors elaborate the study in detail that makes the evaluation and
analysis of medical literature easy for not only the editors and
reviewers, but also for the readers and researchers [4].
TABLE I Reporting Guidelines for Different Types of Study Designs (Available from http://www.equator-network.org/) [9-17]
|
Type of Study |
Reporting Guidelines |
Website |
|
Randomized trials |
CONSORT (CONsolidatedStandards of
Reporting Trials) |
www.consort-statement.org |
|
Observational studies |
STROBE (Strengthening the Reporting of
Observational Studies in Epidemiology) |
http://strobe-statement.org/,
|
|
RECORD (Reporting of studies Conducted
using Observational Routinely-collected
health Data) [10] |
http://www.equatornetwork.org/reportingguidelines/record/ |
|
Systematic review & Meta-analysis |
PRISMA(Preferred Reporting
Items for Systematic Reviews
and Meta-Analyses ) |
http://prisma-statement.org/ |
|
Case reports |
CARE (Case REports) |
http://www.carestatement.org/ |
|
Qualitative research |
SRQR (Standards of Reporting
Qualitative Research) [11] |
http://www.equatornetwork.org/reportingguidelines/srqr/ |
|
ENTREQ(ENhancingTransparency
in REporting the synthesis of Qualitative
research) [12] |
http://www.equatornetwork.org/reportingguidelines/entreq/ |
|
COREQ (COnsolidated criteria for REportingQualitative
research) [13] |
http://www.equatornetwork.org/reportingguidelines/coreq/
|
|
Diagnostic/ Prognostic studies |
STARD 2015 (STAndards for Reporting
Diagnostic accuracy studies) [14] |
www.stard-statement.org/
|
|
TRIPOD(Transparent Reporting
of a multivariable prediction model for Individual
Prognosis Or Diagnosis)
[15] |
http://www.equatornetwork.org/reportingguidelines/tripod-statement/ |
|
Quality improvement studies |
SQUIRE (Standards for Quality
Improvement
Reporting Excellence)
|
http://www.squirev |
|
Economic evaluations |
CHEERS (Consolidated Health
Economic Evaluation Reporting
Standards) [16] |
http://www.equatornetwork.org/reportingguidelines/cheers/ |
|
Study protocols |
SPIRIT (Standard Protocol
Items: Recommendations
for Interventional Trials) [17] |
http://www.spiritstatement.org/ |
Ethical Considerations, Confidentiality, Clinical
Trial Registry and Funding Information
All the clinical studies involving human subjects
need ethical clearance from the local/institutional ethical
committee/review board before initiation of recruitment of subjects. The
same is applicable for animal studies as well. A declaration about the
ethical clearance is mandatory in virtually every medical journal
specifying the authority from where it has been obtained. The study must
comply with the principles of the Declaration of Helsinki [4] – a set of
ethical principles regarding experimentation involving human subjects
developed for the medical community by the World Medical
Association (WMA) [18]. It was first adopted in 1964 and has undergone
several revisions with the latest one in 2013. The ICMR guidelines on
research on human subjects (available from
http://icmr.nic.in/ethical_guidelines.pdf) can also be utilized for
this purpose [19].
The authors need to maintain patient confidentiality;
hence should refrain from using patients’ names, initials, or hospital
numbers, especially in illustrative material. One must specify the
details regarding obtaining informed consent from the
participants/guardians for inclusion in the study and publication of
clinical details or/and clinical photographs. Assent must be taken from
all the subjects more than 7 years of age and the same must be mentioned
in the manuscript.
Most of the medical journals recommend that all
clinical trials involving human subjects to be registered in a public
trial registry before the onset of patient enrolment, and hence also
publish the trial registration number in the manuscript at the end of
abstract [4]. Clinical trial registration helps in preventing
duplication of research activities, and attempts to prevent selective
reporting of research outcomes. One may access these registries to
acquire knowledge regarding current researches going on in a particular
field. Due to these reasons, International Committee of Medical Journal
Editors (ICMJE) encourages registration of the studies with non-trial
research designs as well, even though it is not mandatory. All the
clinical trials being carried out in India need to be registered in the
Clinical Trials Registry of India (www.ctri.in) which is hosted
by the Indian Council of Medical Research. Alternatively, researchers
may register their trials in one of the following trial registries: http://www.actr.org.au; http://www.clinical
trials.gov; http://isrctn.org;http://www.trialregister.nl/trialreg/index.asp; and http://www.umin.ac.jp/ctr.
Funding information may be specified in the methodology section, though
majority of the journals require a separate declaration regarding
funding to be furnished during manuscript submission.
Population, Sample and Participant Characteristics
Authors must describe the population from which they
have chosen the participants of the study. A description of how the
participants were selected i.e. the type of sampling technique
used (e.g. simple random, stratified, cluster, convenience) is
also important. Subjects for the research may be patients, laboratory
samples, animals, hospital records, etc. Similarly, for a
systematic review or meta-analysis, the subjects will be clinical
studies like randomized controlled trials (RCTs). In case of trials,
provide details regarding process of randomization and allocation of
subjects to the different groups. Specify the methods of allocation
concealment and blinding wherever applicable. One should avoid labeling
the different groups with alphabets (groups A,B) or numbers (groups
1,2); rather stick to names e.g. Immunized group and unimmunized
group, to minimize confusion to the readers [20].
In comparative studies like Case-control studies and
Controlled trials, there is a group of subjects that does not receive an
intervention, receives a placebo or receives a different intervention.
It is equally essential to describe the comparison or the control group
characteristics as the main study group. The manuscript must incorporate
the criteria for selection of controls and the methodology used for
allocation of groups that was used to ensure comparability. The authors
must give criteria used for matching, the method of randomization
(simple, stratified, block, etc.), and technique of allocation
concealment and blinding, wherever applicable [2].
Specify the detailed criteria (e.g. age, sex,
well-defined disease condition) which make the participant eligible to
be included in the study. Studies usually also have some exclusion
criteria – parameters that make a subject ineligible to be a part of the
study even after fulfilling the inclusion criteria. The exclusion
criteria are generally outlaid to avoid bias or due to some feasibility
or ethical issues. There should not be any overlap in the inclusion and
exclusion criteria. In other words, the exclusion criteria can be
applied only on the subjects who already fulfill the inclusion criteria.
For example, in a study on prevalence of celiac disease in adolescents
with anemia, inclusion criteria could be:
"All adolescents (age 10-19 years) residing in a
particular community, and having hemoglobin values below the cut-offs (Hb<12g/dL
in 10-19 y girls and 10-14 y boys and Hb<13g/dL in 15-19 y boys)[Reference]."
Exclusion criteria for this study could be:
"Those having received a blood transfusion or
hematinics in preceding 4 weeks, those with acute illness (e.g.
fever, diarrhea, respiratory tract infection) or known chronic disease
(e.g. chronic liver disease, chronic kidney disease, thalassemia)."
Take care not to write: age <10 years or >19 years or
Hb>13 g/dL as exclusion criteria as these subjects already do not
fulfill the inclusion criteria.
Procedure, Intervention and Outcome Measures
The procedure may be just recording an observation,
recording the response to a questionnaire, carrying out a diagnostic
test or doing an intervention which may be preventive or therapeutic.
Mention the exact details of the intervention applied to the
participants in case of trials; description of the drug, device or
educational program being tested, including the exact dosage,
formulation, schedule and duration. For trials as well as for
observational studies, discuss about the process of collecting
information and data for analysis. Give description of the variables
analyzed, technique and the instruments used in the study. Give the
reference if the technique used in the study has been published
previously or is a well-established, standardized one. If that is not
the case, ensure to describe it well with the exact temporal sequence.
Similarly, one must give the manufacturer’s name and place in
parenthesis if a novel apparatus has been used. For example,
"Fasting blood sample was collected to measure HbA1c
by HPLC (BIO-RAD Germany) and lipid profile (enzymatic method). The
GE-Lunar DPX Pro (GE Healthcare, Wisconsin, USA) was used to measure
body compositin [21]."
Outcome measures or study end points are the
parameters which will fulfill the objectives of the study and are
classified as primary and secondary. Primary outcome, which is generally
single, is the parameter on which the study hypothesis is based and is
the main objective of research. The other outcomes of interest, which
may be more than one, are designated as secondary outcomes. These all
should be clearly defined while writing a manuscript. For example,
"The primary outcome was CPAP failure, defined as
need for intubation and mechanical ventilation within 72 hours of
initiation of respiratory support……… The secondary outcomes related to
respiratory support were duration of CPAP support, duration of
supplementary oxygen requirement, maximal flow, PEEP and oxygen
requirement, incidence of air leaks and Broncho-pulmonary dysplasia.
Other outcomes included incidence of patent ductus arteriosus,
intraventricular hemorrhage……."[22].
Statistical Analysis
The data management strategy and statistical analysis
technique used for a study must always be provided in sufficient detail,
to the extent that any skilled person having access to the original data
set is able to reproduce the results. The manuscript must include an
account of sample size calculation with its justification as well as
literature citation as appropriate. The computer software used for data
analysis should also be mentioned. The authors should avoid using
generalized statements and must write statements specific to that study
parameters and outcome variables. The statistical tests and the
comparisons must be specified. Common statistical methods may just be
mentioned but advanced or unusual methods must be described or cited
with an appropriate reference. Description of statistical analysis of
the primary outcome must precede that of secondary outcome(s) [20].
What Not to Write in Methods
Methodology must be described in a complete but
concise manner avoiding any unnecessary detail that is irrelevant to the
readers. Methods section should include only that information that was
available at the time of planning of the study, whereas any information
that was collected while carrying out the study should be a part of the
Results [4]. Avoid giving any explanatory information in this section
like background and rationale for using a particular methodology for a
particular study that may be covered under the section on discussion.
Methods section must include only the proposed sample size and not what
was actually achieved. The account of the subjects who were selected by
sampling till the ones who were eventually analyzed, including details
like refusal to give consent, exclusion based on exclusion criteria must
also be covered under the Results and not Methods [3].
To conclude, the section on methods is the foundation
stone of any research being planned or written; hence it must be clear
and elaborate. It should also be sufficiently described for easy
reproducibility as JC Jones said, "Methodology should not be a fixed
track to a fixed destination but a conversation about everything that
could be made to happen." Laying the strong foundation of a robust
Methods section will pave the way for smooth writing of the rest of the
paper. The next write-up in this series will lead the readers about
intricacies in presenting your Results.
Happy Writing!
1. Dewan P, Gupta P. Writing the title, abstract and
intro-duction: looks matter! Indian Pediatr. 2016;53:235-41.
2. Azevedoa LF, Canário-Almeidaa F, Almeida Fonsecaa
J, Costa-Pereiraa A, Winckb JC, Hespanholb V. How to write a scientific
paper - writing the methods section. Rev Port Pneumol. 2011;17:232-8.
3. Shah D. Material and methods: How will I do it?
In: Gupta P, Singh N, editors. How To Write the Thesis and Thesis
Protocol. New Delhi: Jaypee Brothers; 2014. p. 75-82.
4. Recommendations for the Conduct, Reporting,
Editing, and Publication of Scholarly Work in Medical Journals.
Available from: www.icmje.org/recommendations/Accessed February
25, 2016.
5. Kallestinova ED. How to write your first research
paper. Yale J Biol Med. 2011;84:181-90.
6. Maxwell JA. Methods: what will you actually do?
In: Maxwell JA, editor. Qualitative Research Design: An Interactive
Approach, 2nd ed. Thousand Oaks, CA: Sage; 2005. p. 79-104.
7. Röhrig B, du Prel JB, Wachtlin D, Blettner M.
Types of study in medical research. DtschArztebl Int. 2009; 106: 262-8.
8. Porta M, Greenland S, Last JM, editors. A
Dictionary of Epidemiology. 5thed. New York: Oxford University Press;
2008.
9. EQUATOR Network. Oxford: EQUATOR Network.
Available from: www.equator-network.org/reporting-guidelines/.
Accessed February 25, 2016.
10. Benchimol EI, Smeeth L, Guttmann A, Harron K,
Moher D, Petersen I, et al; RECORD Working Committee. The REporting of
studies Conducted using Observational Routinely-collected health Data
(RECORD) Statement. PLoS Med. 2015;12:e1001885.
11. O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook
DA. Standards for reporting qualitative research: a synthesis of
recommendations. Acad Med. 2014;89:1245-51.
12. Tong A, Flemming K, McInnes E, Oliver S, Craig J.
Enhancing transparency in reporting the synthesis of qualitative
research: ENTREQ. BMC Med Res Methodol. 2012;12:181.
13. Tong A, Sainsbury P, Craig J. Consolidated
criteria for reporting qualitative research (COREQ): a 32-item checklist
for interviews and focus groups. Int J Qual Health Care. 2007;19:349-57.
14. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA,
Glasziou PP, Irwig L, et al., For the STARD Group. STARD 2015: An
updated list of essential items for reporting diagnostic accuracy
studies. BMJ. 2015;351:h5527.
15. Collins GS, Reitsma JB, Altman DG, Moons KG.
Transparent reporting of a multivariable prediction model for individual
prognosis or diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med.
2015;162:55-63.
16. Husereau D, Drummond M, Petrou S, Carswell C,
Moher D, Greenberg D, et al. Consolidated Health Economic
Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ.
2013;14:367-72.
17. Chan A, Tetzlaff JM, Altman DG, Laupacis A,
G¸tzsche PC, Krleþa-Jeriã K, et al. SPIRIT 2013 Statement:
Defining Standard Protocol Items for Clinical Trials. Ann Intern Med.
2013;158:200-7.
18. World Medical Association. World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 2013;310:2191-4.
19. Ethical guidelines for biomedical research on
human participants. New Delhi: Indian Council of Medical Research; 2006.
Available from: http://icmr.nic.in/ethical_guidelines.pdf.
Accessed March 17, 2016.
20. Wenzel V, Dünser MV, Lindner KH. A step by step
guide to writing a scientific manuscript. Available from:
www.aaeditor.org/StepByStepGuide.pdf. Accessed February 23, 2016.
21. Parthasarthy L, Chiplonkar S, Khadilkar V,
Khadilkar A. Association between metabolic control and lipid parameters
in Indian children with type 1 diabetes. Indian Pediatr. 2016;53:39-41.
22. Goel S, Mondkar J, Panchal H, Hegde D, Utture A, Manerkar S.
Nasal mask versus nasal prongs for delivering nasal continuous positive
airway pressure in preterm infants with respiratory distress: a
randomized controlled trial. Indian Pediatr. 2015;52:1035-40.

