|
|
|
Indian Pediatr 2016;53:
221-224 |
 |
Fractional Exhaled
Nitric Oxide for Identification of Uncontrolled Asthma in
Children
|
|
Raj Kumar Meena, Dinesh Raj, Rakesh Lodha and S K
Kabra
From Department of Pediatrics, All India Institute of
Medical Sciences, Ansari Nagar, New Delhi , India.
Correspondence to: Professor SK Kabra, Division of
Pulmonology, Department of Pediatrics, All India Institute of Medical
Sciences, Ansari Nagar, New Delhi-110 029, India.
[email protected]
Received: June 22, 2015; Initial review: August 20,
2015; Accepted: January 14, 2016 .
|
Objectives: To determine the utility of Fractional Exhaled Nitric
Oxide (FENO) in the identification of uncontrolled asthma in children on
therapy, and to identify its cut-off value for determining asthma
control.
Methods: 207 children (age 5-15 y) with
physician-diagnosed asthma on therapy with at least 12 months follow up
were enrolled. Spirometry and FENO measurements were performed. Asthma
control was assessed as per GINA guidelines. Sensitivity and specificity
of various cut-off values of FENO (15 ppb, 20 ppb, 25 ppb, 30 ppb) for
identification of status of control of asthma were calculated.
Results: 156 (75%) children had uncontrolled or
partly controlled asthma and 51 children were assessed to have
controlled asthma. Median (IQR) FENO in children with controlled and
uncontrolled asthma was 16 (11-23) ppb and 13 (11-25) ppb, respectively
(P=0.26). No FENO cut-off had a reasonable combination of
sensitivity and specificity to discriminate between controlled and
uncontrolled asthma.
Conclusion: FENO, in itself, does not have good
discriminatory value in assessment of controlled and uncontrolled asthma
in children on asthma therapy.
Keywords: Airway inflammation, Asthma control, Diagnosis,
Spirometry.
|
|
A
sthma is the commonest chronic respiratory
disorder encountered in clinical practice in children. Standard
guidelines are available which help in monitoring of asthma based on
clinical assessment and spirometry [1]. Clinical assessment may have
problems of under- or over-reporting, and presence of a normal
spirometry does not necessarily establish asthma control. Fractional
exhaled nitric oxide (FENO), which is an indirect evidence of airway
inflammation, has recently been suggested to help in guiding routine
management of asthma [2]. As the target of asthma management is control
of airway inflammation and FENO is a surrogate of airway inflammation,
it is imperative to relate FENO to asthma control.
FENO is a simple non-invasive test and can be easily
measured in pediatric office practice. FENO measurements decrease in a
dose dependent fashion in response to treatment with inhaled
corticosteroids (ICS) [3,4]. FENO has been shown to correlate with the
degree of airway hyper-responsiveness, and the numbers of eosinophils in
induced sputum [5]. Few studies have evaluated the relationship between
asthma control as per Global Initiative for Asthama (GINA) guidelines
and FENO measurements [6-8]. Data are limited from India on the utility
of FENO in management of childhood asthma [9]. We prospectively
evaluated the utility of FENO in identification of uncontrolled asthma
in selected Indian children with asthma. The secondary objective was to
identify cut-off value of FENO for determining asthma control.
Methods
This cross-sectional study was carried out in the
Pediatric Chest Clinic of a tertiary-care hospital in Northern India
over 19 months. Children aged between 5-15 years with
physician-diagnosed asthma, on treatment with a regular follow up of at
least 12 months, were enrolled in the study after obtaining informed
consent from parents. Children not able to perform spirometry and
those with acute exacerbation of asthma (any severity) were excluded
from the study.
Details of history and physical examination were
recorded in a structured form. FENO was measured by using NIOX Mino (Aerocrine
AB, Solna, Sweden) portable machine using standard guidelines [2].
Spirometry was performed using portable spirometer (Superspiro MK2 Micro
Medical Ltd, UK) as per American Thoracic Society (ATS) guidelines [10].
Asthma control was assessed as per GINA guidelines [1]. The study was
approved by the Institutional ethics committee of our institute.
From the existing information, it was expected that
90% of the partly controlled/uncontrolled asthma would have FENO of
³20 ppb.
Required sample size for estimating this sensitivity with a precision of
7.5% and confidence level of 95% was 63. From the data of our Pediatric
Chest Clinic (unpublished), we expected 30% of asthmatics might be
partly controlled/uncontrolled. Therefore, we needed to screen 210
children with asthma to identify 63 children with partly
controlled/uncontrolled asthma.
Statistical analysis: Statistical analysis was
performed using Stata 9.0 statistical software. Number of children with
uncontrolled or partly controlled asthma with exhaled NO
³20 ppb were
calculated to determine the sensitivity. For the purpose of analysis,
uncontrolled asthma included both partly controlled and uncontrolled
children as per GINA guidelines. Receiver operating characteristics
(ROC) curves were constructed using various cut-off values of FENO and
optimal cut off value was calculated. A P value of less than 0.05
was considered significant.
Results
A total of 207 children with asthma were enrolled in
the study. Median (IQR) FENO was 14 (10, 23) ppb. Minimum FENO was 5 ppb
(<5 value was considered equal to 5 ppb) and maximum FENO value was 110
ppb. Seventy-one (34.3%) children had an FENO value of 20 ppb or above.
Based on the GINA guidelines, out of 207 study participants, 156 (75%)
had uncontrolled or partly controlled asthma. Fifty-one (25%)
participants were assessed to have controlled asthma. Median (IQR) FENO
in children with controlled asthma was 16 (11, 23) ppb as compared to 13
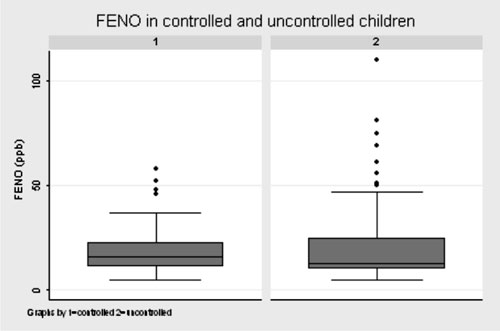
(11, 25) ppb for uncontrolled asthma (P=0.26) (Fig 1).
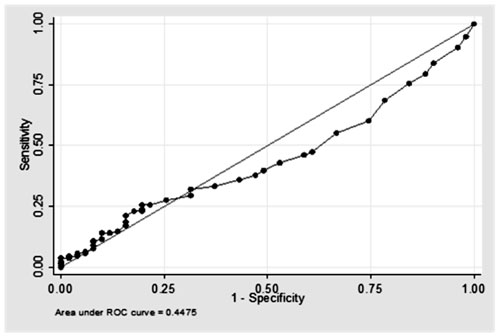
The area under the curve was 0.448, suggesting poor discriminatory value
of FENO for control of asthma (Fig. 2). Table I
shows sensitivity and specificity of various cut-off values of FENO
(15 ppb, 20 ppb, 25 ppb, 30 ppb) for identification of uncontrolled
asthma. None of the cut-off values had a good discriminatory value to
identify asthma control. We compared the characteristics of children
with controlled and uncontrolled asthma (Table II). The
groups did not differ in terms of baseline variables (age, sex, family
history of asthma or allergy). Uncontrolled children had poorer lung
function and were more likely to be using higher doses of inhaled
corticosteroids (Table II).
TABLE I Different Cut-offs of FENO for Assessment of Uncontrolled Asthma
|
FENO cut-off value |
Sensitivity (%) |
Specificity (%) |
|
³15 ppb |
46.2 |
41.9 |
|
³20 ppb |
33.3 |
62.7 |
|
³25 ppb |
25.6 |
80.4 |
|
³30 ppb |
21.2 |
84.3 |
TABLE II Comparison of Characteristics of Children with Controlled and Uncontrolled Asthma
|
Controlled, n=51 |
Uncontrolled, n=156 |
P value |
|
Age, mean (SD) |
120.5 (34.4) |
123.0 (37.0) |
0.67 |
|
Males, n (%) |
35 (68.6) |
119 (76.3) |
0.27 |
|
Family history of asthma or allergy, n (%) |
34 (66.7) |
94 (60.3) |
0.66 |
|
ICS doses |
|
|
|
|
Low, n (%) |
40 (78.4) |
95 (60.9) |
0.02 |
|
High, n (%) |
11 (21.6) |
61 (39.1) |
|
|
FENO, median (IQR) |
16 (11-23) |
13 (11-25) |
0.26 |
|
FENO < 20 ppb, n (%) |
32 (63) |
104 (67) |
0.609 |
|
FENO ³20 ppb, n (%) |
19 (37) |
52 (33) |
|
|
FEV1 % predicted, mean (SD) |
97.8 (13.0) |
87.3 (18.2) |
0.0002 |
|
PEFR % predicted, mean (SD) |
91.2 (17.1) |
75.5 (19.8) |
<0.0001 |
|
FEV1/FVC ratio % predicted, mean (SD) |
101.1 (10.9) |
97.9 (11.0) |
0.0681 |
|
Family history of asthma or allergy, n (%) |
34 (66.7) |
94 (60.3) |
0.6692 |
 |
|
Fig. 1 Box-plot graph showing the
distribution of FENO values in children with controlled and
uncontrolled asthma.
|
 |
|
Fig. 2 ROC curve at different cut off
values of FENO.
|
Discussion
In this cross-sectional study on 207 children, we
observed that FENO has a poor discriminatory power to differentiate
between controlled and uncontrolled/partly controlled asthma as assessed
by GINA guidelines. Earlier studies have evaluated the utility of FENO
measurements in assessing asthma control, and there is evidence in
support [11-15] as well as against [16-19] agreement of FENO with
different measures of asthma control.
GINA guidelines assess control over the preceding
four weeks. On the other hand, FENO measurement is a reflection of
inflammation on the day of assessment. There is a possibility that a
measure of control which takes into account the previous four weeks, and
FENO measurement which assesses inflammation on the day of assessment,
may not show good agreement, as shown by our study.
Corticosteroids (either inhaled or systemic) are
known to bring down FENO levels. A recent study of two distinct
populations (United States and Spain) showed that the correlation
between asthma control (as defined by Asthma Control Test [ACT]) and
FENO was only observed for the Spain site in ICS-naive patients [16].
They concluded that lack of correlation of ACT with FENO probably
reflects the heterogeneity of asthma patients who have varied asthma
severity and treatment regimens. A longitudinal study in unselected
asthmatic patients by Michils, et al. [20] showed that FENO was a
useful marker of asthma control for those patients treated with low
doses of ICS but not for patients on high-to-medium ICS. They suggested
that changes in FENO values, rather than absolute cut-off points (i.e.
personalized FENO profiles), may be more useful. This suggests that ICS
doses might have to be taken into account when using FENO to assess
asthma control.
Khalili, et al. [7] assessed the correlation
between FENO and asthma control (using 5 different asthma control
evaluation tools) in 100 asthmatics (children and adult). No significant
association was found between FENO level and asthma control based on
Asthma Control Questionnaire (ACQ) (P=0.99), ACT (P=0.53),
National Asthma Education and Prevention Program (NAEPP) (P=0.53),
Joint Task Force Practice parameter (JTFPP) (P=0.30), or GINA (P=0.86)
criteria. However, they concluded that commonly used asthma control
evaluation tools do not accurately reflect the status of airway
inflammation as reflected by FENO, and use of such tools may lead to
inappropriate clinical decision making and result in suboptimal
short-term and long-term care. In our study, FENO had a poor sensitivity
and specificity in predicting asthma control. An earlier study noted a
weak but positive correlation between FENO and not well-controlled
asthma [19]. Yavuz, et al. [21] evaluated the role of the C-ACT
and FENO in identifying children with not well-controlled asthma. C-ACT
score of £22
had 69% sensitivity and 77% specificity in identifying not
well-controlled asthma, whereas an FENO value of
³19 ppb had 61%
sensitivity and 59% specificity in patients with at least 3 visits.
The present study has certain limitations. Various
factors/comorbidities affecting FENO measurements including atopy,
allergic rhinitis, eczema, sleep-disordered breathing, and allergen
exposure were not measured and adjusted for in the analysis. The shorter
exhalation used for FENO measurements of younger children (5-8 years)
may also have caused bias.
To summarize, FENO measurement does not seem to have
good discriminatory value in assessment of controlled and uncontrolled
asthma in children between 5-15 years of age on treatment for asthma.
Monitoring of children with asthma should routinely include standard
asthma control tools and spirometry. Measurement of FENO may give useful
information regarding airway inflammation but cannot be used as a
surrogate for asthma control.
Contributors: RKM: study design, data collection,
analysis and manuscript writing, DR: data analysis and manuscript
writing, RL: study design, analysis and manuscript writing; SKK: study
design, data analysis, and manuscript writing. He will act as guarantor
for the study.
Funding: M/s Aerocrine provided material for
carrying out this research. M/s Aerocrine did not have any role in study
design, data collection, data analysis or manuscript writing;
Competing interests: None stated.
|
What is Already Known?
•
Asthma control can be objectively documented with Fractional
exhaled nitric oxide (FENO) values
What This Study Adds?
•
FENO values do not have
discriminatory value for identification of asthma control using
GINA guidelines for assessment of asthma.
|
References
1. From the Global Strategy for Asthma Management and
Prevention, Global Initiative for Asthma (GINA) 2015. Available from:
http://www.ginasthma.org/. Accessed 13.10.2015
2. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh
MW, Lundberg JO, et al., on behalf of ATS Committee on
Interpretation of Exhaled Nitric Oxide (FENO) for Clinical Application.
An official ATS Clinical Practice Guideline: Interpretation of Exhaled
Nitric Oxide Levels (FENO) for clinical applications. Am J Respir Crit
Care Med 2011;184:602-15.
3. Kharitonov SA, Donnelly LE, Montuschi P, Corradi
M, Collins JV, Barnes PJ. Dose-dependent onset and cessation of action
of inhaled budesonide on exhaled nitric oxide and symptoms in mild
asthma. Thorax. 2002;57:889-96.
4. Jones SL, Herbison P, Cowan JO, Flannery EM,
Hancox RJ, McLachlan CR, et al. Exhaled NO and assessment of
anti-inflammatory effects of inhaled steroid: dose-response
relationship. Eur Respir J. 2002;20:601-8.
5. Jatakanon A, Lim S, Kharitonov SA, Chung KF,
Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils,
and methacholine responsiveness in patients with mild asthma. Thorax.
1998;53:91-5.
6. Visitsunthorn N, Prottasan P, Jirapongsananuruk O,
Maneechotesuwan K. Is fractional exhaled nitric oxide (FeNO) associated
with asthma control in children? Asian Pac J Allergy Immunol.
2014;32:218-25.
7. Khalili B, Boggs PB, Shi R, Bahna SL. Discrepancy
between clinical asthma control assessment tools and fractional exhaled
nitric oxide. Ann Allergy Asthma Immunol. 2008;101:124-9.
8. Waibel V, Ulmer H, Horak E. Assessing asthma
control: symptom scores, GINA levels of asthma control, lung function,
and exhaled nitric oxide. Pediatr Pulmonol. 2012;47:113-8.
9. Raj D, Lodha R, Mukherjee A, Sethi T, Agrawal A,
Kabra SK. Fractional exhaled nitric oxide in children with acute
exacerbation of asthma. Indian Pediatr. 2014;51:105-11.
10. Miller MR, Hankinson J, Brusasco V, Burgos F,
Casaburi R, Coates A, et al. Standardisation of spirometry. Eur
Respir J. 2005;26:319-38.
11. Volbeda F, Broekema M, Lodewijk ME, Hylkema MN,
Reddel HK, Timens W, et al. Clinical control of asthma associates
with measures of airway inflammation. Thorax. 2013;68:19-24.
12. Ozier A, Girodet PO, Bara I, Tunon de Lara JM,
Marthan R, Berger P. Control maintenance can be predicted by exhaled NO
monitoring in asthmatic patients. Respir Med. 2011;105:989-96.
13. Delgado-Corcoran C, Kissoon N, Murphy SP,
Duckworth LJ. Exhaled nitric oxide reflects asthma severity and asthma
control. Pediatr Crit Care Med. 2004;5:48-52.
14. Meyts I, Proesmans M, De Boeck K. Exhaled nitric
oxide corresponds with office evaluation of asthma control. Pediatr
Pulmonol. 2003;36:283-9.
15. Sippel JM, Holden WE, Tilles SA, O’Hollaren M,
Cook J, Thukkani N, et al. Exhaled nitric oxide levels correlate
with measures of disease control in asthma. J Allergy Clin Immunol.
2000;106:645-50.
16. Bernstein JA, Davis B, Alvarez-Puebla MJ, Nguyen
D, Levin L, Olaguibel JM. Is exhaled nitric oxide a useful adjunctive
test for assessing asthma? J Asthma. 2009;46:955-60.
17. Green RJ, Klein M, Becker P, Halkas A, Lewis H,
Kitchin O, et al. Disagreement between common measures of asthma
control in children. Chest. 2013; 143:117-22.
18. Ito Y, Adachi Y, Itazawa T, Okabe Y, Adachi YS,
Higuchi O, et al. Association between the results of the
childhood asthma control test and objective parameters in asthmatic
children. J Asthma. 2011;48:1076-80.
19. Vijverberg SJ, Koster ES, Koenderman L, Arets HG,
van der Ent CK, Postma DS, et al. Exhaled NO is a poor marker of
asthma control in children with a reported use of asthma medication: a
pharmacy-based study. Pediatr Allergy Immunol. 2012;23:529-36.
20. Michils A, Baldassarre S, Van Mvylam A. Exhaled
nitric oxide and asthma control: A longitudinal study in unselected
patients. Eur Respir J. 2008;31:539-46.
21. Yavuz ST, Civelek E, Sahiner UM, Buyuktiryaki AB,
Tuncer A, Karabulut E, et al. Identifying uncontrolled asthma in
children with the childhood asthma control test or exhaled nitric oxide
measurement. Ann Allergy Asthma Immunol. 2012;109:36-40.
|
|
|
 |
|

