|
|
|
Indian Pediatr 2014;51:
265-272 |
 |
300,000 IU or 600,000 IU of Oral Vitamin D3
for Treatment of Nutritional Rickets:
A Randomized Controlled Trial
|
|
Hema Mittal, Sunita Rai, Dheeraj Shah, *SV Madhu,
#Gopesh Mehrotra,
$Rajeev
Kumar Malhotra and Piyush Gupta
From Departments of Pediatrics, *Endocrinology,
#Radiology and $Biostatistics; University College of Medical Sciences,
Dilshad Garden, New Delhi 110 095, India
Correspondence to: Professor Piyush Gupta, Block R6A,
Dilshad Garden, New Delhi 110 095, India.
Email:
prof.piyush.gupta@gmail.com
Received: October 25, 2013;
Initial review: December 09, 2013;
Accepted: February 05, 2014.
|
Objective: To evaluate the non-inferiority of a lower therapeutic
dose (300,000 IU) in comparison to standard dose (600,000) IU of Vitamin
D for increasing serum 25(OH) D levels and achieving radiological
recovery in nutritional rickets.
Design: Randomized, open-labeled, controlled
trial.
Setting: Tertiary care hospital.
Participants: 76 children (median age 12 mo)
with clinical and radiologically confirmed rickets.
Intervention: Oral vitamin D3 as 300,000 IU
(Group 1; n=38) or 600,000 IU (Group 2; n=38) in a single
day.
Outcome variables: Primary: Serum 25(OH)D, 12
weeks after administration of vitamin D3; Secondary: Radiological
healing and serum parathormone at 12 weeks; and clinical and biochemical
adverse effects.
Results: Serum 25(OH)D levels [geometric mean
(95% CI)] increased significantly from baseline to 12 weeks after
therapy in both the groups [Group 1: 7.58 (5.50–10.44) to 16.06
(12.71–20.29) ng/mL, P<0.001]; Group 2: 6.57 (4.66–9.25) to 17.60
(13.71–22.60, P<0.001]. The adjusted ratio of geometric mean
serum 25(OH)D levels at 12 weeks between the groups (taking baseline
value as co-variate) was 0.91 (95% CI: 0.65–1.29). Radiological healing
occurred in all children by 12 weeks. Both groups demonstrated
significant (P<0.05) and comparable fall in the serum
parathormone and alkaline phosphatase levels at 12 weeks. Relative
change [ratio of geometric mean (95% CI)] in serum PTH and alkaline
phosphatase, 12 weeks after therapy, were 0.98 (0.7–1.47) and 0.92
(0.72–1.19), respectively. The serum 25(OH)D levels were deficient (<20
ng/mL) in 63% (38/60) children after 12 weeks of intervention [Group 1:
20/32 (62.5%); Group 2: 18/28 (64.3%)]. No major clinical adverse
effects were noticed in any of the children. Hypercalcemia was
documented in 2 children at 4 weeks (1 in each Group) and 3 children at
12 weeks (1 in Group 1 and 2 in Group 2). None of the participants had
hypercalciuria or hypervitaminosis D.
Conclusion: A dose of 300,000 IU of vitamin D3 is
comparable to 600,000 IU, administered orally, over a single day, for
treating rickets in under-five children although there is an
unacceptably high risk of hypercalcemia in both groups. None of the
regime is effective in normalization of vitamin D status in majority of
patients, 3 months after administering the therapeutic dose.
Key Words: Children, Rickets, Treatment, Serum 25(OH)D, Stoss
Therapy, Vitamin D3,
|
|
Indian infants and adolescents
have a high prevalence of hypovitaminosis D and nutritional rickets
[1-3]. Treatment includes therapeutic doses of vitamin D and calcium. In
USA, Australia, and UK, the recommended dose and duration of vitamin D
therapy is variable with either a high dose bolus therapy (Stoss therapy
200,000-600,000 IU of vitamin D as a single oral or parenteral dose or
intermittent high doses) or continuous slow supplementation [4-6]. These
recommendations are based on expert opinion and experience rather than
sound evidence from randomized comparison of doses. Recent guidelines by
Endocrine society recommend 50,000 IU weekly for 6 weeks [7].
Traditionally in India, a single dose of 600,000 IU of vitamin D is used
for treating nutritional rickets in children [8]; no national
recommendations exist. Recent reports have raised concerns of
hypercalcemia following a therapeutic dose of 600,000 IU of vitamin D
[9-11].
Scientific literature to provide evidence for the
best therapy at minimum effective dose – that is feasible, economical
and free of potential adverse effects – is scarce. The evidence for
establishing the minimum effective therapeutic oral dose of vitamin D is
limited to only two trials from Turkey [9,12]. These studies concluded
equal efficacy of single oral dose of 300,000 IU of vitamin D in
comparison to 600,000 IU for treating nutritional rickets in children.
However, these studies had small sample size and did not measure vitamin
D status. Vitamin D metabolism is influenced by several factors,
including availability of sunlight and sun exposure, skin pigmentation,
genetics, and socioeconomic status. Geographical location of Turkey
(39.5ºN) carries a higher risk for hypovitaminosis D due to inadequate
sunshine as compared to India (28ºN), sub-Saharan Africa, Latin America
and Caribbean which have sufficient sunlight throughout the year [13].
Therefore, the results of these trials may not be applicable to tropical
countries in Asia or Africa.
We hypothesized that an oral dose of 300,000 IU of
vitamin D3 is not inferior to 600,000 IU for increasing serum 25(OH)D
levels and achieving radiological recovery for treatment of vitamin D
deficiency rickets of nutritional origin in children between 6 months
and 5 years of age, in a tropical setting.
Methods
This non-inferiority randomized controlled trial was
conducted at a tertiary care hospital attached to a medical school at
Delhi, India from November 2010 to April 2012. A clearance from the
institutional ethical committee and informed consent from parents were
obtained.
Participant selection: Children between the ages
of 6 months and 5 years presenting to pediatric outpatient or emergency
with a combination of clinical evidence of rickets (wide wrists, bow
legs, frontal bossing, rachitic rosary etc.) and radiological findings
(fraying, splaying, and cupping at the epiphyseal ends of long bones in
wrist/knee) consistent with the diagnosis of nutritional rickets
[4,8,14] were eligible for inclusion. Critically ill children and those
having coexisting fat malabsorption, liver or renal insufficiency and
hypercalcemia were excluded. Children with history of having received
vitamin D, calcium supplements, or other medications affecting vitamin D
metabolism (e.g.; anticonvulsants, steroids, cancer chemotherapy)
in previous 6 months were also excluded.
Data collection: Baseline assessment included a
detailed socio-demographic and clinical history and physical examination
at the time of enrolment. Anthropometry (weight, height/length, head
circumference) was recorded as per standard techniques [15]. WHO Child
Growth Standards were used as reference population [16]. The Z-scores
for anthropometric parameters were calculated for each child using the
"WHO Anthro software for PC" [17]. X–rays of the wrist and knee
were obtained for all participants at enrolment, as per standard
procedures [18]. At enrolment, a venous blood sample was obtained for
the estimation of serum calcium, serum phosphorus, serum alkaline
phosphatase, serum 25(OH)D, serum parathormone (PTH), serum albumin,
serum glutamate amino transaminase (SGPT) and serum creatinine.
Randomization and Intervention: Randomization was
done by block randomization (18 blocks of 4 each and 2 blocks of 2
participants each) to 300,000 IU or 600,000 IU of oral vitamin D3 in a
single day. Allocation concealment was done by sealed envelope
technique. Each dose consisted of vitamin D3 [cholecalciferol D 3
(C27H44O);
Mankind Pharma Limited, Delhi, India] in granular form dissolved
completely in 30 mL of milk. The doses were given at an interval of 2
hours under direct supervision (SR) and all children were kept in
hospital for 48 hours, or 24 hours after the last dose of the study
medication; whichever was later. During the stay, participants were
monitored for adverse effects, including vomiting, irritability,
headache, crying, abdominal distension, rash and hypertension. At
discharge, all children were advised to continue calcium supplementation
(30-50 mg/kg/d) orally for 12 weeks. At the end of the study (12 weeks),
all children were advised oral vitamin D3 supplementation (400-1000 IU
daily) for next 3 months.
Follow-up: All children were asked to report for
follow-up at 1 week (±3 d), 4 weeks (±1 week), and 12 weeks (±2 weeks)
after enrolment. At each visit, an interval history was obtained for
adverse effects such as headache, vomiting, abdominal pain, seizures or
bulging fontanelle. A urine sample (5 mL) for estimation of urinary
calcium to creatinine ratio was collected at 1 and 12 weeks after
enrolment. At 4 weeks, a venous blood sample (2 mL) was taken for serum
calcium estimation. Serum 25(OH)D and PTH levels were estimated at
baseline and 12 weeks; simultaneous samples were also drawn for serum
calcium, serum phosphorus and serum alkaline phosphatase. X-rays of the
wrist and knee were repeated at 12 weeks. Follow-up was ensured by
telephone or by personal visit.
Serum samples for 25(OH)D and PTH were stored at –20º
C and analyzed at completion of study. Commercial kits using
radioimmunoassay methods by using gamma counter were used for estimation
of serum 25(OH)D (DiaSorin Inc, USA; interassay variation: 11%;
intra-assay variation: 12.5%) and PTH (Immunotech SAS, France;
interassay variation: 10.3%; intra-assay variation: 7.7%). Serum 25(OH)D
levels were categorized as deficient: <20 ng/mL and normal:
³20ng/mL [7].
Hypocalcemia and hypophosphatemia were defined as serum calcium <8.8 mg/dL
and serum phosphorus <3.8 mg/dL, respectively [19].
Outcome measures: Serum levels of 25(OH)D,
measured 12 weeks after intervention, served as the primary outcome
variable. Secondary outcome variables included radiological healing,
improvement in the radiological severity scores, serum parathormone
level and proportion of children with normal serum alkaline phosphatase.
Serum alkaline phosphatase was measured using Liquick Cor ALP (PZ
CORMAY.SA) and normal levels were defined as per age categories (1–12
months: 82–283 U/L, 13–36 months: 104–345 U/L, >37 months: 93–309 U/L).
PTH levels between 10–65 pg/mL at 12 weeks following administration of
therapeutic dose of vitamin D3 were considered normal. Radiological
healing and severity scores were calculated as described by Thacher,
et al. [20]. Children were categorized as having mild (score
£4), moderate (score
5-8), and severe radiological changes (score >8).
Adverse effects – clinical (headache, vomiting,
abdominal pain, seizures, symptoms of pseudotumor cerebri) and
biochemical (hypercalcemia, urine calcium/creatinine ratio,
hypervitaminosis D) – were also compared between the two groups.
Hypervitaminosis D was defined as level greater than 150 ng/mL [20] and
hypercalcemia was defined as serum calcium greater than 10.8 mg/dL [19].
A urine calcium-creatinine ratio of more than 2 was considered as sign
of toxicity [21].
Sample size: Our past experience suggested that
the serum level of 25(OH)D (primary outcome) follows log-normal
distribution. Sample size for this study was thus calculated on the
basis of geometric mean (GM) ratio and decided to set non-inferiority
lower boundary for mean GM ratio to be 0.8 at 12 weeks. The geometric
mean (GM) ratio indicates geometric mean of 25(OH)D in Group 1 divided
by GM of 25(OH)D in Group 2. Using the coefficient of variation as 0.31
with one-sided 2.5% level of significance and 80% power, a sample size
of 34 subjects in each group was considered adequate. Co-efficient of
variation was calculated on results of a previous study by Soliman,
et al. [22] assuming mean (SD) level of 28.2 (8.7) ng/mL of 25(OH)D
after 3 months of receiving 10,000 IU/kg (maximum 150,000 IU) of vitamin
D. Adding expected 10% lost to follow-up, 38 children per group were
considered appropriate for this non-inferiority trial.
Statistical analysis: The baseline data were
presented in mean and standard deviation for continuous variables and in
proportions for categorical variables. Natural log transformation was
applied for 25(OH)D, serum parathormone, and alkaline phosphatase
because of skewed distribution of these parameters; results were
presented in geometric mean and their 95% confidence intervals.
Two factor repeated measures analysis of variance
(ANOVA) was applied to compare baseline and 12 weeks levels of serum
25(OH)D, PTH, calcium, phosphorus, and alkaline phosphatase. The
interaction between the group and time was tested. Analysis of
covariance (ANCOVA) was applied to compare the serum 25(OH)D levels at
12 weeks between the groups taking baseline value as covariate, and data
expressed as geometric mean and 95% confidence intervals for log
transformed variable; and mean ± SD for other continuous variables.
Homogeneity of slope assumption of analysis of covariance was tested by
including the interaction (group × baseline value). The value of serum
25(OH)D, serum parathormone, and alkaline phosphatase were expressed as
relative change due to log transformation and calcium, phosphorus, and
alkaline phosphatase were expressed as mean change. The least square
means (estimated marginal means) resulting from the ANCOVA was used to
calculate the one side 97.5% confidence interval for log transformed
difference between the two treatment groups. The acceptable lower limit
of one-sided 97.5% confidence interval of serum 25(OH)D level at dose
300,000 IU was set at 80% of that achieved in the group receiving
600,000 IU of vitamin D3. Intention to treat analysis was also used in
full-analysis set considering baseline value as carried forward at 12
weeks.
Analysis was done with SPSS-20 (Chicago, IL).
Results
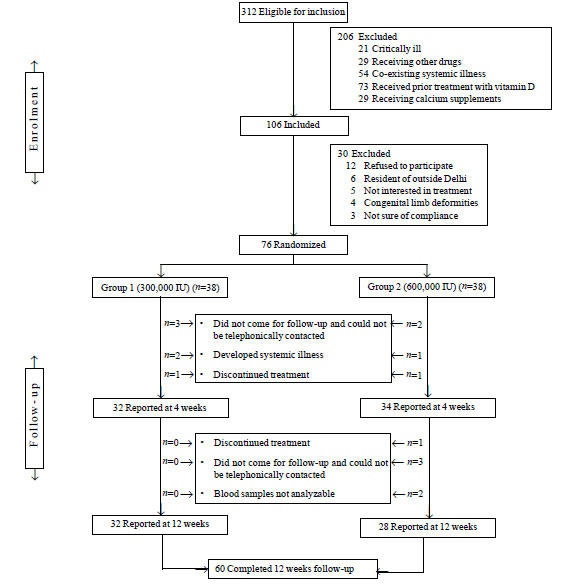
Enrolment and baseline characteristics: A total
of 312 children with rickets were diagnosed over 18 months, of which 106
met the inclusion criterion. Fig. 1 shows the enrolment
and follow-up of children assessed in the study. Of the 76 children
enrolled, 45% (n=34) were females. Baseline demographic,
clinical, and biochemical features; and radiological score of children
in the two Groups are shown in Table I.
 |
|
Fig.1 Participant flow diagram.
|
TABLE I Baseline Clinical and Biochemical Characteristics of Enrolled Children
|
Parameter |
Group 1 |
Group 2 |
|
300,000 IU
|
600,000 IU |
|
(n=38) |
(n=38) |
|
(Mean±SD) |
(Mean±SD) |
|
Age (months) |
15.8 ±13.23 |
19.1 ±14.40 |
|
Weight-for-age (Z score ) |
-2.8 ±1.82 |
-2.9 ±1.60 |
|
Length-for-age (Z score)
|
-2.3 ± 1.80 |
-2.9 ± 2.48 |
|
Weight-for-length (Z score ) |
-0.6 ±1.64 |
-0.1 ± 1.7 |
|
Serum vitamin D (ng/mL) |
10.5 ± 9.91 |
9.5 ± 6.9
|
|
Loge (Serum vitamin D)
|
1.96 ± 0.92 |
1.95 ± 0.84 |
|
Serum parathormone
|
166.6±151.86 |
110.8 ±112.17 |
|
Loge (Serum PTH) |
4.53±1.27 |
4.05±1.25 |
|
Serum ALKPO4(U) |
981.5±518.4 |
1096.9±1035.2
|
|
Loge (Serum ALKPO4) |
6.77±0.50 |
6.74± 0.70 |
|
Serum calcium (mg/dL) |
7.7±1.29 |
7.7±1.19 |
|
Serum phosphorus (mg/dL) |
3.5± 1.29 |
3.3± 1.31 |
|
ALKPO4: alkaline phosphatase; vitamin D: 25(OH)D; PTH:
parathormone. |
The presenting complaints included not gaining height
(n=43, 56.6 %), irritability (n=30, 39.5%), delayed gross
motor development (n=29, 38.2%), bowing of legs (n=19,
25%) hypocalcemic convulsions (n=13, 17 %), bone pains (n=4,
5.5 %), delayed tooth eruption (n=1, 1.3%) and recurrent
infections (n=61, 80.3%). Recurrent infections included
respiratory tract infections (n=52) and diarrhea (n=9).
On examination, most frequent clinical findings were
wrist widening (n=76, 100%), frontal bossing (n=72,
94.7%), protruded abdomen (n=64, 84%), rachitic rosary (n=62,
81.6%), Harrison sulcus (n=60, 79%), genu varum (n=46,
61.8%), genu valgum (n=1, 1.3%), wide open anterior fontanel (n=29,
38.7%), hypotonia of limbs (n=4, 5.5%), delayed tooth eruption (n=3,
3.9%) and enamel defects (n=2, 2.5%).
Biochemical abnormalities included raised alkaline
phosphatase (n=76,100%), hypocalcemia (n=63, 82.9%) and
hypophosphatemia (n=47, 61.8%). The median value (IQR) of serum
alkaline phosphatase, serum phosphorus and serum calcium were 843.50
(582–333.5); 3.2 (2.5–4.3) mg/dL, and 7.8 (6.8–8.5) mg/dL, respectively.
Serum parathormone levels were elevated (>65 pg/mL) in 43 (56.6%)
children at enrolment. Serum 25(OH)D levels were categorized as normal,
and deficient in 8 (10.5%) and 68 (89.5) children, respectively.
Radiological evidence of rickets included fraying and
splaying in all children (n=76, 100%). Osteopenia, cupping and
loss of demarcation between metaphysis and epiphysis were seen in 67% (n=51)
of children. Mild, moderate and severe radiological changes were present
in 29 (38.1%), 6 (7.9%), and 41 (54%) children, respectively. The
distribution was comparable between the two groups (data not shown).
TABLE II Comparison Between Baseline and 12 Weeks Value of Biochemical Variables
|
Variable |
Baseline |
12 Weeks# |
Relative change |
P-value(interaction
|
|
Geometric mean |
Geometric mean |
from baseline: |
between time and
|
|
(95% CI) |
(95% CI) |
(95% CI) |
Groups) |
|
Serum 25(OH)D (ng/mL) |
|
G-1 |
7.58 (5.50-10.44) |
16.06 (12.71-20.29) |
2.12 (1.42-3.17) |
0.42 |
|
G-2 |
6.57 (4.66-9.25) |
17.60 (13.71-22.60) |
2.68 (1.73-4.13) |
|
|
Serum parathormone (pg/mL) |
|
G-1 |
80.24 (50.30-127.99) |
24.15 (18.19-32.06) |
0.30 (0.20-0.46) |
0.19 |
|
G-2 |
46.40 (28.73-78.09) |
21.59 (16.72-27.88) |
0.47 (0.28-0.78) |
|
|
Serum alkaline phosphatase (U/L) |
|
G-1 |
851.3 (693.0-1045.2) |
347.2 (289.8-416.6) |
0.41(0.37-0.50) |
0.48 |
|
G-2 |
809.2 (654.6-1000.3) |
369.8 (306.7-446.3) |
0.46 (0.35-0.59) |
|
|
Serum calcium (mg/dL) |
|
G-1 |
7.54 ± 1.38 |
9.14 ± 0.68 |
*1.62 (1.07-2.18) |
0.68 |
|
G-2 |
7.64 ± 1.15 |
9.08 ± 1.03 |
*1.47(0.98-1.96) |
|
|
Serum phosphorous (mg/dL) |
|
G-1 |
3.55 ± 1.25 |
4.59 ± 1.08 |
*1.04 (0.44-1.64) |
0.77 |
|
G-2 |
3.79 ± 3.66 |
4.65 ± 1.11 |
*0.86 (-0.30-2.01) |
|
|
*Mean change (12 weeks – base line) (95% CI);
G-1: Group-1(Vitamin D 300,000 IU; n=32); G-2: Group-2 (Vitamin
D 600, 000 IU; n=28). Analysis of variance used for within group
and between group differences. All differences are unadjusted;
#P value <0.001 for within group changes for all variables,
except serum phosphorus where P = 0.003.
|
Outcome measures: The changes in biochemical
parameters in the two groups after 12 weeks of enrolment are compared in
Table II. Children in both the groups doubled their
baseline serum 25(OH)D level. Serum parathormone and alkaline
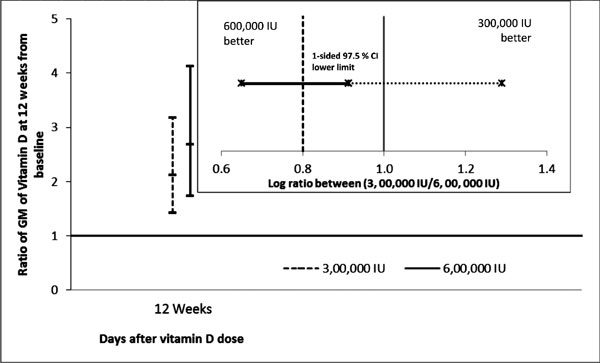
phosphatase declined by approximately 60%. The adjusted ratio of
geometric mean of serum 25(OH)D at 12 weeks between the Groups (taking
baseline value as covariate) was 0.91 (95% CI: 0.65-1.29) (Table
III).This is also diagrammatically depicted in Fig. 2.
The results were almost similar when intention to treat analysis (ITT)
approach was applied with baseline observations carried forward for
25(OH)D.
TABLE III Comparison of Biochemical Parameters Between the Groups, 12 Weeks After Intervention
|
Variable |
*Estimated marginal geometric means (95% CI)
|
Relative change[ratio of
|
|
Group 1 (n=32) |
Group 2 (n=28) |
geometric means
|
|
(300,000 IU)
|
(600,000 IU) |
(95% CI)] |
|
Serum 25(OH)D (ng/mL) |
16.1 (12.68-20.35) |
17.6 (13.67-22.67) |
0.91 (0.65-1.29) |
|
Serum parathormone (pg/mL) |
23.1 (17.98-29.73) |
22.7 (17.34-29.73) |
0.98 (0.70-1.47) |
|
Serum alkaline phosphatase (U/L) |
345.2 (290.3-407.5) |
372.8 (312.0-445.4) |
0.92 (0.72-1.19) |
|
Serum calcium (mg/dL) |
#9.2 (8.88-9.50) |
#9.1 (8.71-9.38) |
$0.14 (-0.311-0.598) |
|
Serum phosphorous (mg/dL) |
#4.6 (4.20-4.98) |
#4.7 (4.23-5.07) |
$-0.06 (-0.629-0.515) |
|
*Analysis of covariance with baseline value adjusted as covariate; Homogeneity of slope assumption is fulfilled for all the five variables (P>0.05);
# Estimated marginal means (95% CI); $Mean change (95% CI) |
 |
|
Fig. 2 Ratio of geometric means
from baseline to 12 weeks (main graph) and mean ratio between
the doses (inset graph).
|
At 12 weeks, all children in both the groups
demonstrated evidence of radiological healing. The mean (SD)
radiological severity scores improved significantly (P<0.001) in
both the groups after 12 weeks of intervention [Group 1: 2.3 (1.25);
Group 2: 2.6 (1.31)]. Improvement in scores was comparable between the
groups at 12 weeks. There was no child in either group with radiological
severity score >8; only one child in Group 1 and 2 children in Group 2
had radiological severity score 5-8; and 31 children in Group 1 and 26
children in Group 2 had severity scores
£4, respectively.
Almost half the children in both the groups showed
normalization of alkaline phosphatase levels after receiving vitamin D3
treatment at 12 weeks [Group 1: 17/32 (53%); Group 2: 15/28 (54%)].
Secondary hyperparathyroidism (serum PTH >65 pg/mL) was present in 65.8%
(25/38) children of Group 1 and 47.4% (18/38) children of Group 2 at
enrolment. At 12 weeks, only 4 children in Group 1 and one child in
Group 2 had raised serum PTH levels. Web Fig. 1
shows the distribution of children categorized according to serum
25(OH)D levels (both at baseline and 12 weeks after therapy).
Web
Fig. 2 shows the increase in serum 25(OH)D in each
child in both the groups after 12 weeks of treatment.
Adverse effects: No child required a repeated
dose of study medication or developed any signs of drug intolerance
(nausea, vomiting, headache, persistent crying, etc.). No clinical
adverse effects of Vitamin D3 therapy or infections were noticed in both
the groups. During first week, 5 children had diarrhea (Group 1: 2,
Group 2: 3). Four children developed respiratory tract infection in
second week (Group 1: 2, Group 2: 2).
Laboratory parameters showed no signs of raised
urinary calcium creatinine ratio at 1 and 12 weeks. The mean (SD)
urinary calcium creatinine ratio in both the groups was comparable
[Group 1: 1.2 (1.03), Group 2: 1.2 (0.86); P =0.92]. At 12 weeks
also, all children had normal urinary calcium creatinine ratio, and mean
(SD) levels were comparable in both the groups [Group 1: 1.0 (0.81),
Group 2: 0.9 (0.7); P =0.8].
No child had evidence of hypervitaminosis D.
Hypercalcemia was documented in 2 children (1 in each Group) at 4 weeks;
and 3 children (1 child in Group 1 and 2 children in Group 2) at 12
weeks.
Discussion
In this study on 76 children (6 mo–5 y) with
nutritional rickets, a therapeutic oral dose of 300,000 IU of vitamin D3
was comparable to 600,000 IU for improving 25(OH)D status after 12 weeks
of its administration. All children in both the groups demonstrated
radiological healing at 12 weeks. Also, there was comparable improvement
in radiological severity scores, and comparable decline of serum
parathormone and alkaline phosphatase levels in both the treatment arms.
We concluded that 300,000 IU of vitamin D3 is not inferior to 600,000 IU
for treating nutritional rickets in under-five children. There is a
potential risk of hypercalcemia with both regimes.
Our study had certain limitations. The diagnosis of
rickets was on clinical and radiological parameters: biochemical indices
were not included. Seven (9.2%) children were vitamin D sufficient
(serum 25(OH)D >20 ng/mL) but had clinical and radiological evidence of
rickets. A previous study by Voloc, et al. [7] has demonstrated
poor correlation between clinical features and serum 25(OH)D levels.
Radiological changes in rickets may also be due to hypocalcemia in face
of sufficient serum 25(OH)D levels [26]. We did not differentiate
between calcium deficient rickets and vitamin D-deficient disease. We
also did not study the effect of vitamin D3 therapy on clinical
improvement (nutritional status, reversal of deformities). A valid
interpretation of our results on adverse effects is also not possible
due to limitation of small sample size for adverse effects as an outcome
measure. We did not estimate serum 25(OH)D levels at 1 week after
therapy, which would have been a better indicator of immediate toxicity.
There was smaller increase in 25(OH)D levels in our
study (10.8 ng/mL) as compared to that by Soliman, et al (21.95
ng/mL) [22]. Lower increase in mean levels of 25(OH)D may be related to
complex mechanisms in pharmacokinetics and pharmacodynamics of vitamin D
leading to difference in individual responses, altered vitamin D
metabolism in Asian Indians [23], and vitamin D receptor (VDR)
polymorphism or mutations [24]. It is also concluded that increase in
serum 25OH vitamin D levels are dependent on baseline levels, dose of
vitamin D, and weight of patients [25]. Due to a small
post-supplementation increase in serum 25(OH)D levels, proportion of
children having 25(OH)D level between 5–20 ng/mL remained almost same,
before and after treatment.
Our results demonstrate that even a dose of 600,000
IU may not be enough to normalize serum 25(OH)D (beyond 20 ng/mL), in
Indian children with rickets. We advised daily vitamin D supplementation
for all children for 12 weeks, after completing the study duration of 3
months. We wonder, whether these children should have been started with
routine daily vitamin D supplementation, immediately following the mega
dose. This approach would have needed a strict monitoring for vitamin D
intoxication.
We conclude that a therapeutic oral dose of 300,000
IU of vitamin D can be safely substituted for 600,000 IU for treating
nutritional rickets in under-five children. None of the two regimes is
effective in normalization of vitamin D status in majority of patients,
3 months after administering the therapeutic dose. Studies are needed to
document the optimal strategy of vitamin D3 supplementation in children
treated with mega-dose of vitamin D, for replenishing the body stores.
Contributors: The study was conceptualized
by PG. Methodology was finalized with inputs from DS, SR, SVM, GM, and
RKM. Data were collected by SR and HG. Laboratory support and
interpretation was provided by SVM. GM was responsible for assessment
and interpretation of radiological results. The manuscript was drafted
by HG and SR with inputs from SVM, GM, and RKM. Statistical analysis was
planned and conducted by PG and RKM. PG and DS critically reviewed the
manuscript for intellectual content. All authors approved the final
paper. PG shall stand as the Guarantor.
Funding: Indian Council of Medical Research, New
Delhi; and University College of Medical College; Delhi.
Competing interests: None stated.
References
1. Seth A, Marwaha RK, Singla B, Aneja S, Mehrotra
P, Sastry A, et al. Vitamin D nutritional status of exclusively
breast fed infants and their mothers. J Pediatr Endocrinol Metab.
2009;22:241-6.
2. Balasubramanian S, Ganesh R. Vitamin D deficiency
in exclusively breast-fed infants. Indian J Med Res. 2008;127: 250-5.
3. Marwaha RK, Sripathy G. Vitamin D and bone mineral
density of healthy school children in northern India. Indian J Med Res.
2008;127:239-44.
4. Mishra M, Pacaud D, Petryk A, Solberg PF, Kappy M.
Vitamin D deficiency in children and its management: review of current
knowledge and recommendations. Pediatrics. 2008;122:398-417.
5. Muuns C, Zacharin MR, Rodda CP, Batch JA, Morley
R, Cranswick NE, et al. Prevention and treatment of infant and
childhood vitamin D in Australia and New Zealand: A consensus statement.
Med J Aust. 2006;185:268-72.
6. Davies JH, Shaws NZ. Preventable but no strategy:
vitamin D deficiency in the UK. Arch Dis Child. 2011;96:614-5.
7. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon
CM, Hanley DA, Heaney RP et al; Endocrine Society. Evaluation,
treatment, and prevention of vitamin D deficiency: an Endocrine Society
clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-30.
8. Gupta P, Shah D, Ghai OP. Micronutrients in health
and disease. In: Ghai OP, Gupta P, Paul VK (editors). Essential
Pediatrics, 6th ed. New Delhi: CBS; 2004. P.129.
9. Ozkan B, Buyukavcï M, Energin M. Nutritional
rickets: comparison of three different therapeutic approach 300,000 U
p.o., 300,000 IU i.m. and 600,000 IU p.o. Cocuk Sagöligöive Hastaliklari
Dergisi. 2000;43:30-5.
10. Billoo AG, Murtaza G, Memon MA, Khaskheli SA,
Iqbal K, Rao MH. Comparison of oral versus injectable vitamin D for the
treatment of nutritional vitamin D deficiency rickets. J Coll Physicians
Surg Pak. 2009;19:428-31.
11. Voloc A, Esterle L, Nguyen TM, Debray OW,
Colofitchi A, Jehan F, et al. High prevalence of genu varum/valgum
in European children with low vitamin D status and insufficient dairy
products/calcium intakes: Clinical study. Eur J Endocrinol.
2010;163:811-7.
12. Cesur Y, Caksen H, Gundem A. Comparison of low
and high dose of vitamin D treatment in nutritional vitamin D deficiency
rickets. J Pediatr Endocrinol Metab. 2003;16:1105-9.
13. Arabi A, Rassi RE, Fuleihan GE. Hypovitaminosis D
in developing countries – prevalance, risk factors and outcomes. Nat Rev
Endocrinol. 2010;6:550–61.
14. Shah D, Gupta P. Nutrition and health. In:
Gupta P, editor. Textbook of Pediatrics. New Delhi: CBS Publishers and
Distributors; 2013.p.51-82.
15. WHO. Physical status: The Use and Interpretation
of Anthropometry. Report of WHO Expert Committee. Geneva: WHO; 1987.
16. World Health Organization. The WHO Child Growth
Standards. Available from: http://www.who.int/childgrowth/en/. Accessed
on September 11, 2012.
17. WHO Anthro for Personal Computers, Version 3.2.2,
2011: Software for Assessing Growth and Development of the World’s
Children. Geneva: WHO, 2010. Available from:
http://www.who.int/childgrowth/software/en/. Accessed on September 11,
2012.
18. Merill V. Atlas of Roentgenographic Positions and
Standard Radiologic Procedures. Fourth Edition. USA: Mosby; 1975.p. 38
and 92.
19. Nicholson JF, Pesce MA. Reference ranges for
laboratory tests and procedures. In: Behrman RE, Kliegman RM,
editors. Nelson Textbook of Pediatrics. 17th edition.
Philadelphia: Saunders; 2003. p. 2396-427.
20. Thacher TD, Fischer PR, Pettifor JM, Lawson JO,
Manaster BJ, Reading JC. Radiographic scoring method for the assessment
of the severity of nutritional rickets. J Trop Pediatr. 2000;46:132–9.
21. Holick MF. The role of vitamin D for bone health
and fracture prevention. Curr Osteoporos Rep. 2006;4:96–102.
22. Soliman AT, Dabbagh M, Adel A, Ali MA, Bedair EM,
Alaily RK. Clinical responses to a mega-dose of vitamin D3 in infants
and toddlers with vitamin D deficiency rickets. J Trop Pediatr.
2010;56:19-26.
23. Awumey EM, Mitra DA, Hollis BW. Vitamin D
metabolism is altered in Asian Indians in the southern United States: a
clinical research study. J Clin Endocrinol Metab. 1998;83:169-73.
24. Vupputuri MR, Goswami R, Gupta N, Ray D, Tandon
N, Kumar N. Prevalence and functional significance of 25-hydroxyvitamin
D deficiency and vitamin D receptor gene polymorphisms in Asian Indians.
Am J Clin Nutr. 2006;83:1411-9.
25. Garg MK, Marwaha RK, Khadgawat R, Ramot R, Oberoi
AK, Mehan N, et al. Efficacy of vitamin D loading doses on serum
25-hydroxy vitamin D levels in school going adolescents: an open label
non-randomized prospective trial. J Pediatr Endocrinol Metab.
2013;26:515-23.
26. Khadgawat R, Marwaha RK, Garg MK, Ramot R,
Oberoi AK Sreenivas V, et al. Impact of vitamin D fortified milk
supplementation on vitamin D status of healthy school children aged
10-14 years. Osteoporos Int. 2013;2:2335-343.
27. Sahay M, Sahay R. Rickets-vitamin D deficiency and dependency.
Indian J Endocrinol Metab. 2012;16:164-76.
|
|
|
 |
|

