|
|
|
Indian Pediatr 2013;50:
383-389 |
 |
Bilirubin Nomogram for Prediction of
Significant Hyperbilirubinemia in North Indian Neonates
|
|
Umesh Pathak, Deepak Chawla, Saranjit Kaur and Suksham Jain
From Department of Pediatrics, Government Medical
College and Hospital, Chandigarh, India.
Correspondence to: Dr Deepak Chawla, Assistant
Professor, Department of Pediatrics, Government Medical College and
Hospital, Chandigarh, India.
Email:
[email protected]
Received: March 29, 2012;
Initial review: May 22, 2012;
Accepted: October 04, 2012.
Published online: 2012, October 5.
PII: S097475591200290
|
Objectives: (i) To construct hour-specific serum total
bilirubin (STB) nomogram in neonates born at
≥35
weeks of gestation; (ii)To evaluate efficacy of pre-discharge
bilirubin measurement in predicting hyperbilirubinemia needing
treatment.
Design: Diagnostic test performance in a
prospective cohort study.
Setting: Teaching hospital in Northern India.
Subjects: Healthy neonates with gestation
³35
weeks or birth weight ³2000
g.
Intervention: Serum total bilirubin was measured
in all enrolled neonates at 24±6, 72-96 and 96-144 h of postnatal age
and when indicated clinically. Neonates were followed up during hospital
stay and after discharge till completion of 7th postnatal day.
Outcome: Key outcome was significant
hyperbilirubinemia (SHB) defined as need of phototherapy based on
modified American Academy of Pediatrics (AAP) guidelines. In neonates
born at 38 or more weeks of gestation middle line and in neonates born
at 37 or less completed weeks of gestation, lower line of phototherapy
thresholds were used to initiate phototherapy. For construction of
nomogram, STB values were clubbed in six-hour epochs (age ± 3 hours) for
postnatal age up to 48 h and twelve-hour epochs (age ± 6 hours) for age
beyond 48 h. Predictive ability of the nomogram was assessed by
calculating sensitivity, specificity, positive predictive value,
negative predictive value and likelihood ratio, by plotting
receiver-operating characteristics (ROC) curve and calculating
c-statistic.
Results: 997 neonates (birth weight: 2627 ± 536
g, gestation: 37.8±1.5 weeks) were enrolled, of which 931 completed
followup. Among enrolled neonates 344 (34.5%) were low birth weight.
Rate of exclusive breastfeeding during hospital stay was more than 80%.
Bilirubin nomogram was constructed using 40th, 75th and 95th percentile
values of hour-specific bilirubin. Pre-discharge STB of
≥95th
percentile was assigned to be in high-risk zone, between 75th and 94th
centile in upper-intermediate risk zone, between 40th and 74th centile
in lower-intermediate risk zone and below 40th percentile in low-risk
zone. Among 49 neonates with pre-discharge STB in high risk zone. 34
developed SHB (positive predictive value: 69.4%, sensitivity: 17.1%,
positive likelihood ratio: 8.26). Among 342 neonates with pre-discharge
STB in low risk zone, 32 developed PHB (negative predictive value: 90.6%
and specificity: 42.5%, positive likelihood ratio: 0.37). Area under
curve for this risk assessment strategy was 0.73.
Conclusion: Hour-specific bilirubin nomogram and
STB measurement can be used for predicting subsequent need of
phototherapy. Further studies are needed to validate performance of risk
demarcation zones defined in this hour-specific bilirubin nomogram.
Key words: Diagnostic test, Jaundice, Neonate, Outcome.
|
|
A significant proportion of
neonates develop hyperbilirubinemia needing treatment (‘significant’
hyperbilirubinemia, SHB) during first week of life [1]. Decrease in
duration of birth hospitalization has been temporally associated with
increased incidence of bilirubin induced neurological damage [2].
Post-discharge home visits by health worker or hospital visits by family
may detect SHB, but are not universally feasible or cost-effective.
Therefore, before neonates are discharged from birth hospital those at
risk of developing high bilirubin levels need to be identified [3]
Pre-discharge objective assessment for risk of
developing SHB is also important because of limited accuracy of visual
assessment of extent of jaundice [4]. Risk stratification for SHB has
been done by measuring bilirubin load (absolute levels or rate of rise
of serum total bilirubin or transcutaneous bilirubin), bilirubin
production (exhaled carbon monoxide) and identifying underlying clinical
risk factors [5-7].
The concentration of bilirubin in peripheral blood is
a function of age-specific rates of bilirubin production, metabolism,
excretion and reabsorption. Therefore, interpretation of bilirubin level
in a neonate is based on postnatal age. Hour-specific bilirubin nomogram
developed by Bhutani, et al. [8] has demonstrated that
measurement of serum total bilirubin (STB) before discharge from birth
hospital can help in identifying neonates who are at risk of having
higher percentile values of STB during followup. Among various risk
prediction methods pre-discharge measurement of STB has shown best
discriminating ability among North American neonates [9-10]. However,
due to genetically determined differences in bilirubin metabolism and
dissimilarities in feeding practices clinical course of
hyperbilirubinemia may vary in neonates belonging to different
ethnicities or geographic locations. In addition, the previous nomogram
was developed from a retrospective cohort in whom the information about
outcome of significant hyperbilirubinemia was known for only one-fifth
of study population [8]. Therefore, construction of hour-specific
bilirubin nomogram in different neonatal populations is a prerequisite
for using pre-discharge bilirubin measurement as a risk assessment
strategy.
We planned this prospective cohort study to construct
hour-specific serum total bilirubin nomogram in Indian neonates and to
evaluate efficacy of pre-discharge bilirubin measurement in predicting
hyperbilirubinemia needing treatment among term and late-preterm
neonates.
Methods
This prospective cohort study with evaluation of
diagnostic test performance was conducted from February to June 2010 at
a teaching hospital in northern India. Study protocol was approved by
Ethics Committee of the hospital and written informed consent was
obtained from parents. Healthy neonates with gestation
≥35 weeks or birth
weight ≥2000 g
were eligible for enrolment in the study. Due to logistic reasons,
neonates completing 24 h of life on Sunday were not eligible for
enrolment in the study. Neonates with major congenital malformation,
admission in neonatal intensive care unit, positive direct Coombs’ test
(was done if mother blood group was Rhesus negative), phototherapy
before first bilirubin measurement or inability to come for follow-up
were excluded.
Study measurements and follow-up: Blood sample
for first measurement of serum total bilirubin was withdrawn at the time
of metabolic screening at 18-30 h of postnatal age. Capillary or
peripheral venous blood was collected in pre-heparinized
micro-capillaries. Blood was centrifuged immediately at 12000 rpm for 5
minutes and total bilirubin was measured with a spectrophotometer
(NEO-BIL plus, das srl, Italy).
Neonates were followed up during hospital stay and
after discharge till completion of 7th postnatal day. The timing of
follow-up visit was decided based on age at discharge. Babies discharged
before 48 h of age were called back between 72 and 96 h of age and
babies discharged after 48 h of age at 96 to 120 h of age. In addition
to first measurement of STB at the time of metabolic screening, two more
STB measurements were performed in each neonate. After first
measurement, decision to perform second and third STB measurements was
based on clinical assessment. Clinical assessment of degree of jaundice
was accompanied by transcutaneous bilirubin (TcB) measurement with a
multi-wavelength transcutaneous bilimeter (BiliChek, coefficient of
variation <5%). STB estimation was done if palms/soles were stained with
icterus or TcB was >12 mg/dL or within 80% of age-specific phototherapy
threshold. If not indicated clinically, second and third STB
measurements were done at 72-96 h and 96-144 h of postnatal age,
respectively. STB values after starting phototherapy were not included
for construction of the nomogram.
Clinical and epidemiological risk factors which may
influence risk of developing SHB were recorded. Following data were
recorded: birthweight, gestation, gender, maternal education and
religion, parity, antenatal complications, maternal ABO and Rh blood
group, mode of delivery, type of anesthesia used during delivery and use
of oxytocin infusion during labor. In addition, age at initiation of
feeding, supplemental feeding (other than breast feeding or expressed
breast milk) during and subsequent to first 24 h after birth and age at
passage of first stool were also noted.
Outcome: Key outcome was significant
hyperbilirubinemia (SHB) which was defined as need of phototherapy or
exchange transfusion for treatment of hyperbilirubinemia. The decision
to start phototherapy was made on the basis of the age of the baby in
hours and STB levels, as per local adaptation of American Academy of
Pediatrics (AAP) guidelines [3]. In neonates born at 38 or more weeks of
gestation, medium-risk threshold, and in neonates born at 37 or less
completed weeks of gestation, higher-risk threshold, was used to
initiate phototherapy. Medium-risk threshold values in AAP guidelines
are almost identical to 95 th
percentile values of Bhutani nomogram [3,8].
Statistical analysis: In a prospective study
significant hyperbilirubinemia was observed in 10% of neonates born at
≥35 weeks of
gestation [5]. For investigating a diagnostic test with sensitivity of
at least 95% (confidence interval 5%) and alpha value of 0.05, we needed
to enrol about 1000 subjects [11].
Data were analyzed using Stata 9 (StataCorp, College
Station, TX, USA). For construction of nomogram, STB values were clubbed
in six-hour epochs (age±3 hours) for postnatal age up to 48 h and
twelve-hour epochs (age±6 hours) for age beyond 48 h. Data for each
epoch was examined for symmetry. The 5 th,
10th, 25th,
40th, 75th,
90th and 95th
percentile values were calculated for each epoch. Microsoft Excel
(Microsoft Corporation, Richmond, US) was used to plot the hour-specific
bilirubin nomogram. Smoothened nomogram depicting 40th,
75th and 95th
percentile was plotted using cubic spine modeling with GAMLSS package
for R statistical software. After smoothening 36.5% cases were below 40th
percentile line, 77.8% cases were below 75th
percentile line and 95.1% cases were below 95th
percentile line. Predictive ability of the nomogram was assessed by
calculating sensitivity, specificity, positive predictive value,
negative predictive value and likelihood ratio, by plotting
receiver-operating characteristics (ROC) curve and calculating
c-statistic.
Results
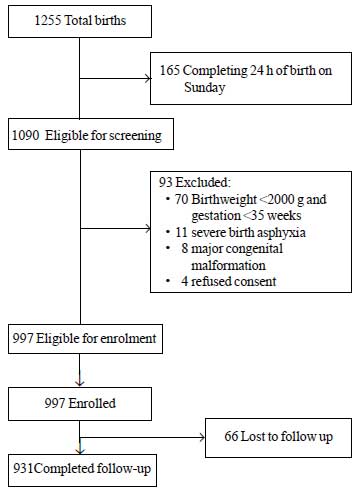
During the study period, a total of 1255 neonates
were born of which 1090 were eligible for enrolment. Among these, 93
were excluded for different reasons (Fig. 1). A total of
997 neonates were enrolled in the study. Mean ± SD value for birthweight
was 2627±536 g and for gestation age was 37.8±1.5 weeks (median and IQR:
38 and 37-39) (Web Table I). Most of study infants were
born after uncomplicated antenatal course and had uneventful transition
to extrauterine life. More than 80% of neonates were breastfed
exclusively during the hospital stay.
 |
|
Fig.1 Study flow.
|
Construction of bilirubin nomogram: First
measurement of bilirubin was performed at 23.3±6.3 h of age and mean STB
was 7.0±2.0 mg/dL. Twenty nine (2.9%) neonates needed phototherapy based
on first measurement of bilirubin. In these neonates phototherapy was
started at 27±5.6 h of age with STB levels of 12.3±2.0 mg/dL. Sixty-six
(6.6%) neonates were lost to follow-up after discharge from study
hospital. First bilirubin value in these neonates was comparable to
neonates who never developed SHB (6.5±1.9 vs 6.7±1.7 mg/dL, P=0.54)
and was significantly lower than those who developed SHB (6.5±1.9 vs
8.5±2.2 mg/dL, P< 0.001).
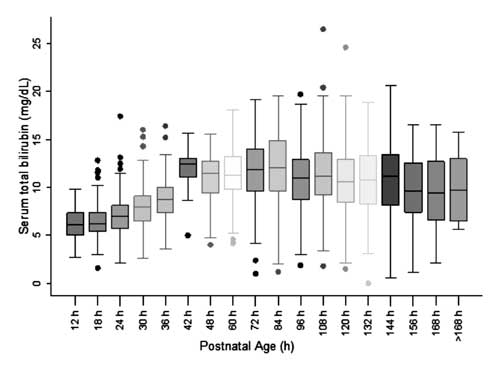
For construction of nomogram and assessing
distribution of STB values, the postnatal age was divided into six-hour
epochs for postnatal age up to 48 h and twelve-hour epochs for age
beyond 48 h. The STB at each of the epochs except at 42 h was observed
to be symmetrically distributed (Fig. 2). Distribution of
STB values at 42 h was observed to be positively skewed and these values
were not used for construction of the nomogram.
 |
|
Fig. 2 Box-whisker plot showing
distribution of serum total bilirubin.
|
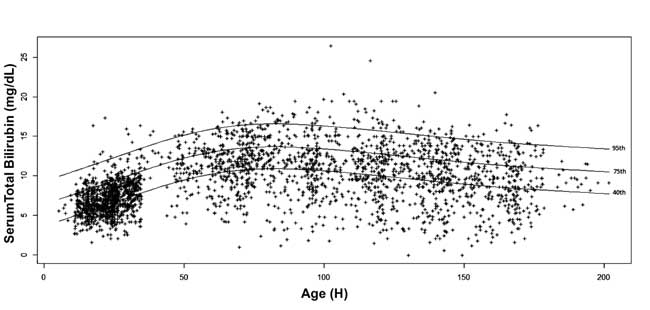
The 5 th,
10th, 25th,
40th, 75th,
90th and 95th
percentile values for each epoch were calculated.
Age-specific serum bilirubin nomogram was drawn with 40th,
75th and 95th
percentile values at advancing postnatal age (Fig. 3).
 |
|
Fig. 3 Bilirubin nomogram -
hour-specific serum total bilirubin depicted as 40th, 75th and
95th percentiles.
|
Predictive ability of pre-discharge STB: Overall,
199 (20%) neonates developed SHB (received phototherapy). First
bilirubin value was used to predict subsequent need of treatment for
hyper-bilirubinemia. If more than two values were obtained in first 48 h
after birth, higher percentile value was used for prediction purpose.
TABLE I Predictive Characteristics of Percentile Values as Risk Demarcators for Subsequent Need of
Treatment for Hyperbilirubinemia
|
Pre-discharge serum total bilirubin |
Outcome |
Test performance |
|
Percentile |
Number(n=928) |
SHB+ |
SHB- |
PPV |
NPV |
Sensitivity |
Specificity |
|
Above 95th percentile |
49 |
34 |
15 |
69.4 |
81.2 |
17.1 |
97.9 |
|
Below 95th percentile |
879 |
165 |
714 |
|
|
|
|
|
Above 75th percentile |
239 |
107 |
132 |
44.8 |
86.2 |
53.8 |
81.9 |
|
Below 75th percentile |
689 |
92 |
597 |
|
|
|
|
|
Above 40th percentile |
586 |
167 |
419 |
28.5 |
90.6 |
83.9 |
42.5 |
|
Below 40th percentile |
342 |
32 |
310 |
|
|
|
|
SHB: significant hyperbilirubinemia, PPV: positive predictive value, NPV: negative predictive value.
|
Among neonates who had pre-discharge STB measurement
and completed follow-up (n=928), in 49 (5.3%) neonates
pre-discharge STB was more than 95th
percentile of age-specific distribution (Table
I). Of these 34 neonates subsequently needed phototherapy (positive
predictive value: 69.4%, sensitivity: 17.1%). In 342 (36.8%) neonates
pre-discharge STB was less than 40th
percentile of age-specific distribution. Of these, 310 neonates did not
need subsequent treatment for hyperbilirubinemia (negative predictive
value: 90.6% and specificity: 42.5%). Positive predictive value of 75th
percentile cut-off was 44.8% and negative predictive value was 86.2%.
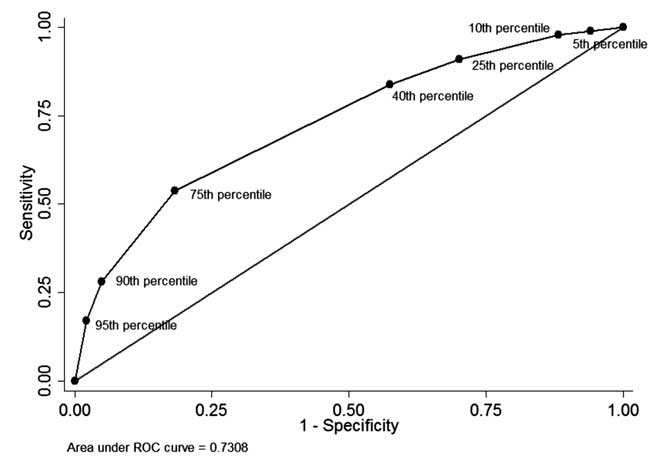
The ROC curve as shown in Fig. 4 illustrates the
diagnostic performance of each percentile-cutoff with area under curve
(c-statistic) being 0.73.
 |
|
Fig. 4 The ROC curve for diagnostic
ability of different percentile cut-offs of pre-discharge serum
total bilirubin.
|
Likelihood ratio (LR) is a better tool of measuring
diagnostic test performance as the ratio is unaffected by change in
background prevalence of the outcome. LR of positive test (LR+,
likelihood of test positive in diseased/likelihood of test positive in
non-diseased) was calculated for each risk demarcation zone.
Pre-discharge STB of ≥95th
percentile was assigned to be in high-risk zone, between 75th
and 94th centile in
upper-intermediate risk zone, between 40th
and 74th centile in
lower-intermediate risk zone and below 40th
percentile in low-risk zone. Among 49 neonates in high-risk zone 34
developed SHB; therefore, positive LR for STB in high-risk zone was 8.26
(Table II). Among 190 neonates in upper-intermediate risk
zone, 73 developed SHB; therefore, positive LR for STB in this risk zone
was 2.30. Similarly, positive LR for STB in lower-intermediate risk zone
was 0.76 and for low-risk zone was 0.37.
TABLE II Predictive Ability of Pre-discharge Serum Total Bilirubin for Subsequent Significant
Hyperbilirubinemia (Need of Phototherapy)
|
Pre-discharge serum total bilirubin |
|
|
Outcome |
|
|
Test performance |
|
|
Pre-discharge cumulative risk zone |
Percentile |
Total |
SHB+ |
SHB- |
P:A ratio |
Probability of disease |
LR+ |
|
High-risk |
≥95th |
49 |
34 |
15 |
7:3 |
7/10 |
8.26 |
|
Upper-intermediate |
75th to 94th |
190 |
73 |
117 |
2:3 |
2/5 |
2.30 |
|
Lower-intermediate |
40th to 74th |
347 |
60 |
287 |
1:5 |
1/6 |
0.76 |
|
Low-risk |
<40th |
342 |
32 |
310 |
1:10 |
1/11 |
0.37 |
|
|
928 |
199 |
729 |
1:4 |
1/5 |
|
|
SHB: significant hyperbilirubinemia; P:A ratio: Presence of outcome : Absence of outcome. |
Discussion
Pre-discharge risk assessment for subsequent
development of SHB is recommended as a potential strategy to reduce the
incidence of bilirubin induced neurological damage or kernicterus. In
this prospective cohort study we have constructed hour-specific serum
bilirubin nomogram in a subset of north Indian neonates and have
evaluated the efficacy of risk demarcation by pre-discharge STB
measurement in predicting subsequent need of phototherapy (SHB).
Baseline incidence of SHB was high in our study cohort with 2 out of 10
neonates developing SHB. Location of pre-discharge STB in two higher
risk zones significantly increased the risk of subsequent SHB with 7 out
of 10 neonates in high risk zone developing SHB (positive LR=8.26) and 4
out of 10 neonates in higher-intermediate risk zone developing SHB
(positive LR=2.3). Location of pre-discharge STB in low risk zone
significantly decreased the risk of subsequent SHB with 1 out of 10
neonates developing SHB (positive LR=0.37). However, as negative
predictive value of low risk cut-off was only 90%, location in low-risk
zone was not able to rule-out the possibility of subsequent SHB.
Bhutani, et al. [8] showed in a large cohort
that neonates with pre-discharge STB in high- and high-intermediate risk
zones are more likely to have SHB during followup. The authors
constructed percentile charts of serum bilirubin level at different
postnatal ages in near-term and term neonates. They found that 6.1% of
neonates had pre-discharge serum bilirubin >95th percentile; 32.1% of
these infants showed hyperbilirubinemia subsequently. In comparison to
hour-specific nomogram by Bhutani, et al. [8], percentile values
of STB in this study are higher by up to 2 mg/dL till 84-108 h of
postnatal age. Neonates of north Indian origin have been observed to
reach higher values of bilirubin and have higher incidence of
hyperbilirubinemia [5]. Mean STB of 7.0±2.0 mg/dL observed in this study
is between 75th and 95th
percentile of Bhutani nomogram. Similarly Agarwal, et al. [5]
reported a mean STB of 5.9±1.8 mg/dL at 24 h of postnatal age which is
close to 75th percentile
value of Bhutani nomogram. Higher proportion of preterm or low birth
weight neonates and higher rate of exclusive breastfeeding in our study
may be the factors contributing to increased STB values and increased
incidence of SHB. In addition, our decision to use middle instead of
upper line of AAP phototherapy thresholds even in low-risk neonates also
increased the incidence of SHB. Beyond 108 h of postnatal age,
percentile values of STB in this study are lower than corresponding
values in the Bhutani nomogram. Inclusion of STB values from neonates
who were selectively followed up on clinician judgement for construction
of nomogram may have resulted in use of higher STB levels for plotting
the Bhutani nomogram, therefore diminishing latter’s generalizability
[12]. In the present study, as follow-up was completed irrespective of
severity of hyperbilirubinemia, the nomogram peaks on 4th
and 5th day of postnatal age
with natural decline at end of the first week.
In a prospective cohort study, Agarwal, et al.
measured STB at 24±6 h of age in 220 neonates born at
≥35 weeks of
gestation for prediction of hyperbilirubinemia [5]. Absence of STB >6
mg/dL at 24±6 h of age virtually ruled out the possibility of subsequent
SHB (likelihood ratio of negative test 0.07) within 5 days of birth.
However, selective measurement of outcome in only those neonates who
during followup had ‘clinical’ bilirubin level of >10 mg/dL introduced
verification bias in the study. In another Indian study, a cut-off of
3.99 mg/dL at 18-24 h was found to have sensitivity and specificity of
67% each for prediction of subsequent bilirubin level >15 mg/dL [13].
However, complete follow-up was present only in infants who stayed in
the hospital either for neonatal illness or some maternal reason, such
as cesarean section. More than 50% of infants, who were healthy and thus
discharged early, were not followed up. A study from Turkey presented
hour-specific bilirubin nomogram in neonates with a gestational age
between 35 and 37 weeks. STB value more than 95 th
percentile had a high positive predictive value for subsequent
development of SHB [14]. However, STB value less than 30th
percentile had a negative predictive value of about 90%. Two large
retrospective studies have reported excellent predictive ability of
early/pre-discharge measurement of STB with area under curve (AUC) of
0.83 [15,16]. In our study,
discriminating ability of 40th
and 75th percentile values
was lower than those previously reported [15,16]. This shifted the ROC
curve in our study towards the diagonal line resulting in decreased
discriminating ability (AUC= 0.73). High baseline incidence of SHB in
Turkish (25.3%) and our study (20%) may explain the inability of low
percentile values to rule-out the development of subsequent SHB, thereby
limiting the utility of pre-discharge STB measurement.
An alternative risk assessment strategy for
prediction of subsequent SHB is evaluation of clinical risk factors.
Gestation at birth, history of jaundice needing treatment in previous
sibling, oxytocin infusion, instrumental delivery, birth trauma and
inadequate feeding have been implicated as risk factors of SHB
[15,17,18]. However,
discriminating ability of clinical risk model has been reported to be
lower than that of early STB measurement [15]. Newman, et al.
[16] reported improved discriminating ability when a clinical risk
instrument was combined with early STB measurement [16]. Due to
significant proportion of low birth weight and preterm neonates in our
cohort, we speculate that combination of these objectively measurable
clinical risk factors with early STB measurement would generate a risk
model with improved discriminating ability.
External applicability of observations made in the
study may be influenced by relatively high incidence of
hyperbilirubinemia in the study cohort because of use of lower bilirubin
thresholds for starting phototherapy. In contrast to developed
countries, kernicterus has been reported at lower levels of peak
bilirubin in India, which indicates that Indian neonates may develop
bilirubin-induced neurological damage at lower peak serum bilirubin
levels [19,20]. In addition, about one-third neonates born in India are
of low birthweight. Owing to these reasons, the National Neonatology
Forum of India in its guidelines suggests use of lower thresholds for
starting phototherapy, especially in areas with higher incidence of
glucose-6-phosphate dehydrogenase deficiency [21-22].
Strengths of our study include prospective study
design, large sample size, more than 90% follow-up rate and absence of
verification bias. We could not ascertain occurrence of outcome in about
7% of enrolled neonates. However, as early STB and demographic
characteristics in these lost-to-follow-up neonates were similar to
those who never developed SHB, hour-specific nomogram and risk
assessment instrument are unlikely to be affected. We did not use high
performance liquid chromatography (HPLC) which is the ‘gold-standard’
method for measurement of bilirubin. We measured bilirubin by a more
commonly used bedside method of spectrophotometry. The bilimeter used in
our study had low coefficient of variation and it was calibrated before
each use.
We recommend that as neonates with pre-discharge STB
in high or high-intermediate risk zone have high probability of
developing SHB early and frequent follow-up should be ensured. In
settings where close follow-up is not feasible, delaying discharge from
hospital till bilirubin falls to lower risk zones may be considered.
Neonates with pre-discharge STB in lower-intermediate or low risk zones
can be discharged as per local policy. However, adequate follow-up
should be ensured as subsequent development of SHB cannot be ruled out.
In conclusion, despite fair discriminating ability,
the higher level of follow-up in our study increases the confidence in
the ability of pre-discharge STB to predict SHB in Indian infants.
Further studies are needed to validate performance of risk demarcation
zones defined in this hour-specific bilirubin nomogram.
Contributors: DC: conceptualized and designed the
study; UP and SK: collected data; DC: analyzed data; UP: drafted the
paper with critical inputs from DC, SK and SJ. All authors approve final
version of manuscript for submission.
Funding: None; Competing interests: None
stated.
References
1. Report 2002-2003: National Neonatal Perinatal
Database Network. New Delhi: National Neonatology Forum of India; 2004.
2. Watchko JF. Identification of neonates at risk for
hazardous hyperbilirubinemia: emerging clinical insights. Pediatr Clin
North Am. 2009;56:671-87.
3. Management of hyperbilirubinemia in the newborn
infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
4. Lodha R, Deorari AK, Jatana V, Paul VK.
Non-invasive estimation of total serum bilirubin by multi-wavelength
spectral reflectance in neonates. Indian Pediatr. 2000;37:771-5.
5. Agarwal R, Kaushal M, Aggarwal R, Paul VK, Deorari
AK. Early neonatal hyperbilirubinemia using first day serum bilirubin
level. Indian Pediatr. 2002;39:724-30.
6. Stevenson DK, Fanaroff AA, Maisels MJ, Young BW,
Wong RJ, Vreman HJ, et al. Prediction of hyperbilirubinemia in
near-term and term infants. Pediatrics 2001;108:31-39.
7. Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin
C, Johnson LH. Noninvasive measurement of total serum bilirubin in a
multiracial predischarge newborn population to assess the risk of severe
hyperbilirubinemia. Pediatrics. 2000;106:E17.
8. Bhutani VK, Johnson L, Sivieri EM. Predictive
ability of a predischarge hour-specific serum bilirubin for subsequent
significant hyperbilirubinemia in healthy term and near-term newborns.
Pediatrics. 1999;103:6-14.
9. Ip S, Chung M, Kulig J, O’Brien R, Sege R, Glicken
S, et al. An evidence-based review of important issues concerning
neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-53.
10. Trikalinos TA, Chung M, Lau J, Ip S. Systematic
review of screening for bilirubin encephalopathy in neonates.
Pediatrics. 2009;124:1162-71.
11. Carley S, Dosman S, Jones SR, Harrison M. Simple
nomograms to calculate sample size in diagnostic studies. Emerg Med J.
2005;22:180-1.
12. Fay DL, Schellhase KG, Suresh GK. Bilirubin
screening for normal newborns: a critique of the hour-specific bilirubin
nomogram. Pediatrics. 2009;124:1203-5.
13. Awasthi S, Rehman H. Early prediction of neonatal
hyperbilirubinemia. Indian J Pediatr. 1998;65:131-9.
14. Sarici SU, Serdar MA, Korkmaz A, Erdem G, Oran O,
Tekinalp G, et al. Incidence, course, and prediction of
hyperbilirubinemia in near-term and term newborns. Pediatrics.
2004;113:775-80.
15. Keren R, Bhutani VK, Luan X, Nihtianova S, Cnaan
A, Schwartz JS. Identifying newborns at risk of significant
hyperbilirubinaemia: a comparison of two recommended approaches. Arch
Dis Child. 2005;90:415-21.
16. Newman TB, Liljestrand P, Escobar GJ. Combining
clinical risk factors with serum bilirubin levels to predict
hyperbilirubinemia in newborns. Arch Pediatr Adolesc Med.
2005;159:113-9.
17. Newman TB, Xiong B, Gonzales VM, Escobar GJ.
Prediction and prevention of extreme neonatal hyperbilirubinemia in a
mature health maintenance organization. Arch Pediatr Adolesc Med.
2000;154: 1140-7.
18. Keren R, Luan X, Friedman S, Saddlemire S, Cnaan
A, Bhutani VK. A comparison of alternative risk-assessment strategies
for predicting significant neonatal hyperbilirubinemia in term and
near-term infants. Pediatrics. 2008;121:e170-9.
19. Murki S, Kumar P, Majumdar S, Marwaha N, Narang
A. Risk factors for kernicterus in term babies with non-hemolytic
jaundice. Indian Pediatr. 2001;38:757-62.
20. Agrawal VK, Shukla R, Misra PK, Kapoor RK, Malik
GK. Brainstem auditory evoked response in newborns with
hyperbilirubinemia. Indian Pediatr. 1998;35:513-8.
21. Kumar P, Jain N, Thakre R, Murki S, Venkataseshan
S (eds). Evidence Based Clinical Practice Guidelines. National
Neonatology Forum of India, New Delhi, India, 2010.
22. Kaur G, Srivastav J, Jain S, Chawla D, Chavan BS,
Atwal R, et al. Preliminary report on neonatal screening for
congenital hypothyroidism, congenital adrenal hyperplasia and
glucose-6-phosphate dehydrogenase deficiency: a Chandigarh experience.
Indian J Pediatr. 2010;77:969-73.
|
|
|
 |
|

