|
|
|
Indian Pediatr 2013;50: 371-376
|
 |
Nasal Intermittent Positive Pressure
Ventilation versus Nasal Continuous Positive Airway
Pressure in Neonates: A Systematic Review and Meta-analysis
|
|
Shifang Tang, Jinning Zhao, Jie Shen, Zhangxue Hu and Yuan Shi
From the Department of Pediatrics, Daping
Hospital, Institue of Surgery Research, Third Military Medical
University, Chongqing 400042, China.
Correspondence to: Dr Yuan Shi, Director and
Professor, Department of Pediatrics, Daping Hospital, Third
Military Medical University, Chongqing 400042, China.
Email:
[email protected]
Received: November 3, 2011;
Initial review: November 28, 2011;
Accepted: September 20, 2012.
Published online: 2012, October 05.
PII: S097475591100912
|
Objective
: To compare the
efficacy and safety of Nasal intermittent positive pressure ventilation
(NIPPV) and Nasal continuous positive airway pressure (nCPAP) in
neonates.
Methods: Standard search strategy for the
Cochrane Neonatal Review Group was performed. The participants were both
preterm and term infants suffering from neonatal respiratory distress
syndrome or experiencing apnea of prematurity.
Results: 14 eligible andomized controlled trials
involving 1052 newborn infants were included. The study quality and
evidence validity was defined as moderate. As compared with nCPAP, NIPPV
significantly reduced the incidence of endotracheal ventilation
(OR=0.44, 95%CI:0.31–0.63), increased the successful rate of extubation
(OR=0.15, 95%CI:0.08–0.31), and had a better outcome indicated by
decreased death and/or bronchopulmonary dysplasia (OR=0.57,
95%CI:0.37–0.88). Moreover, NIPPV decreased the number of apneic
episodes of prematurity (WMD=-0.48, 95%CI:-0.58–0.37), and marginally
decreased the incidence of bronchopulmonary dysplasia (OR=0.63,
95%CI:0.39–1.00). No side effects specifically associated with NIPPV
were reported.
Conclusions: NIPPV could be used to reduce
endotracheal ventilation, increase successful extubation, decrease the
rate of apnea of prematurity, and have better outcome indicated by fewer
death and/or bronchopulmonary dysplasia in preterm and term newborn
infants.
Key words: Management, Mechanical ventilation, Neonate,
Respiratory distress syndrome, Outcome.
|
|
Nasal intermittent
positive pressure ventilation (NIPPV) has been
widely used in neonatal intensive care unit
(NICU) [1]. As a mode of non-invasive
ventilation, NIPPV is suggested to increase the
beneficial effects of nasal continuous positive
airway pressure (nCPAP) and, therefore, decrease
the need for endotracheal intubation. Several
explanations have been put forward for the
mechanism of NIPPV [2-4]. Addition of increased
flow delivery in the upper airway, increased
tidal and minute volumes, increased functional
residual capacity, recruitment of collapsed
alveoli, improved stability of the chest wall,
and less asynchrony of thoraco-abdominal
movement have been shown with the application of
NIPPV in newborn infants [5].
Some meta-analyses on the
comparison of the effect of NIPPV with nCPAP in
neonatal respiratory distress syndrome (NRDS)
were published on Cochrane Database a few years
ago [6-8]. However, only preterm infants were
included in these. Recently, NIPPV has been
further studied in randomized controlled trials.
In addition to major outcome, more data on
bronchopulmonary dysplasia (BPD), retinopathy of
prematurity (ROP), intraventricular hemorrhage
(IVH) and periventricular leukomalacia (PVL)
have been investigated. More studies on the
safety of NIPPV were also reported, which
concerned the incidence of pneumothorax or air
leak, abdominal distention, necrotizing
enterocolitis, and patent ductus arteriosus
(PDA). Hence, it is necessary to systematically
evaluate the effectiveness of NIPPV compared
with nCPAP in NRDS.
Methods
Criteria for inclusion and
exclusion: Studies were included in the
systematic review if they were randomized or
quasi-randomized. The participants were both
preterm and term infants suffering from neonatal
respiratory distress syndrome (NRDS) or
experiencing apnea of prematurity. The
interventions for comparison were NIPPV and
nCPAP. Studies which did not report outcomes
specified in this review were excluded.
NRDS has been suggested not
only to be present in preterm infants but also
in term infants [9]. Studies involved both
preterm and term infants were eligible if there
were clinical evidences of NRDS. The diagnosis
of NRDS was based on clinical manifestation and
X-ray picture [10].
Outcome measures: During
NIPPV versus nCPAP in the post-extubation
period, the major outcome was respiratory
failure leading to endotracheal intubation and
mechanical ventilation. When NIPPV versus
nCPAP were used as a primary respiratory
support, the major outcome was the need of
intubation. The secondary outcomes included the
rate of apnea, the incidence of BPD, ROP, IVH,
PVL, PDA, pneumothorax or air leak, abdominal
distention, necrotizing enterocolitis, the total
stay in the hospital, and the mortality. Final
outcome was determined by the mortality and/or
BPD. A good outcome was defined as the infant
could be discharged without oxygen treatment,
whereas a bad outcome was defined as death
and/or BPD.
Search strategy and methods
of the review: Standard search strategy for
the Cochrane Neonatal Review Group was
performed. Searches were made in PubMed, EMBASE,
Ovid, Springer and China Knowledge Resource
Integrated (CNKI) databases with the terms:
newborn OR preterm AND respiratory distress
syndrome AND nasal intermittent positive
pressure ventilation AND nasal continuous
positive airway pressure. The search time was
from the beginning of the databases to March
2011. Grey literature and conference abstracts
were not searched.
Two reviewers performed
searches and assessed study quality
independently. Study quality was assessed
according to Cochrane Handbook for Systematic
Reviews of Interventions Version 5.0.2, which
included allocation concealment, sequence
generation, blinding of participants, blinding
of researchers, blinding of assessors,
incomplete data address, free of selective
reporting, and free of other bias [11]. If the
article fulfilled all the mentioned criteria, it
was classified as adequate and with the least
possibility of bias. If the article could not
fulfill more than one criterion, it was
classified as highly deflective. Discussions
were made by the reviewers group when there were
different opinions about the evaluation for the
quality of articles.
Data were extracted and
analysed independently by the two reviewers,
following the methods of the Cochrane
Collaboration and using the statistical software
of Review Manager 4.22, then compared, and the
differences resolved.
Statistical analysis: A
chi-square test was used to evaluate the
statistical homogeneity. If P ³0.10,
it was judged as statistically
non-heterogeneous, and a fixed effect model
selected. If P<0.10, it was judged
statistically heterogeneous, and a random effect
model selected. Categorical data were analyzed
using odds ratio (OR) with 95% confidence
intervals (95% CI). Continuous data were
analyzed using means and weighted mean
difference (WMD) with 95% CI. A P value
<0.05 was defined as significant.
Results
On initial search 103
articles were identified, including 97 English
papers and 6 papers in other languages with
English abstracts (4 Chinese, 1 Spanish, 1
Polish). According to the inclusion criteria, 14
randomized controlled trials involving 1052
newborn infants were included (11 English, 3
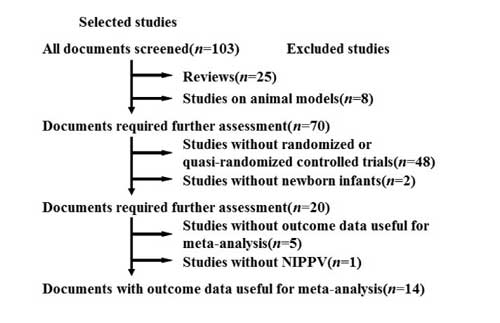
Chinese) [12-25]. The selection course of the
papers was shown as Fig 1. Among
them, 5 trials investigated the effect of NIPPV
versus nCPAP in the post-extubation
period following ETT and mechanical ventilation
[12-16]
(Web Table I). The
other 9 trials studied the effect of NIPPV
versus nCPAP as a primary respiratory
support
[17-25]
(Web Table
II).
 |
|
Fig.1 The
selection course of the papers.
|
The basic data were compared
to understand the clinical homogeneity of the
included studies, which showed a comparable
basic line. Five papers on NIPPV versus
nCPAP as mode of extubation were clinically
heterogeneous in gestational age, birth weight,
regulation data of NIPPV or nCPAP, criteria for
extubation, and criteria for re-intubation. Nine
papers on NIPPV versus nCPAP as a primary
respiratory support had a clinical homogeneity
in the inclusive criteria, regulation data of
NIPPV or nCPAP, and outcome measure, but there
was a little clinically heterogeneity in
gestational age and birth weight, because 2
studies involved both preterm and term infants,
and 1 paper studied late-preterm infants. Most
of the major patients were preterm infants with
low or very low birth weight, and there were not
enough term infants to be analysed in sub-group.
Methodological quality:
The results of the assessment of methodological
quality is shown in Web Fig. 1.
Adequate concealment at randomization, complete
follow-up, and free of selective reporting was
identified in all 14 studies. Thirteen studies
mentioned the sequence generation. Two studies
stated no blinding of researchers.
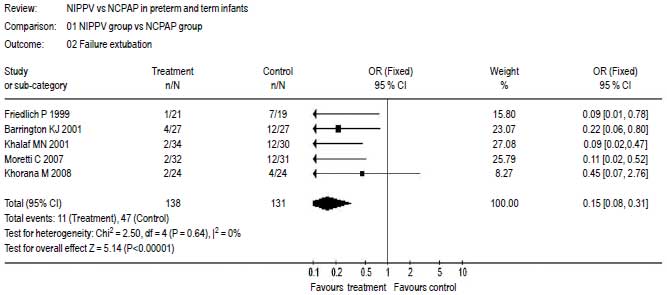
Major outcome: Five
papers [12-16] reported the rate of extubation
failure of NIPPV versus nCPAP following
ETT and mechanical ventilation. The studies were
statistically homogeneous (P=0.64), and a
fixed effect model was selected. Meta-analysis
showed that the rate of extubation failure of
NIPPV was significantly lower than that of nCPAP
[OR=0.15 (95% CI: 0.08 0.31)]; P<0.001 (Fig.
2).
 |
|
Fig.2 The
failure extubation rate of NIPPV versus
nCPAP.
|
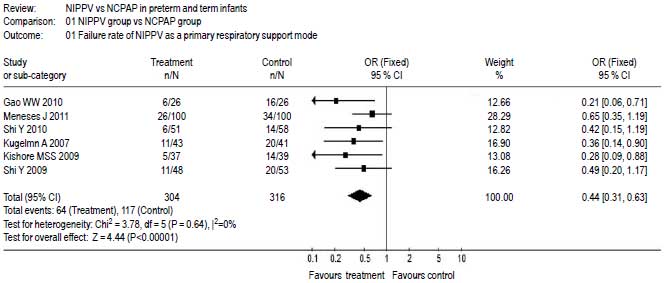
Six papers [20-25] reported
the failure rate of NIPPV versus nCPAP as
a primary respiratory mode, which was indicated
by whether or not requiring ETT and mechanical
ventilation. The results were statistically
homogeneous (P=0.58), and a fixed effect
model was selected. Meta-analysis showed that
the failure rate of not needing needing ETT and
mechanical ventilation in NIPPV group was
significantly lower than that in nCPAP group as
a primary respiratory mode [OR=0.44 (95% CI:
0.31-0.63); P<0.0001) (Fig. 3).
 |
|
Fig.3 The failure rate of
NIPPV versus nCPAP as a primary
respiratory mode. |
Secondary outcome: Five
papers [20-23,25] reported the comparison of
NIPPV and nCPAP on the final outcome as a
primary respiratory mode, which was indicated by
death and/or BPD requiring respiratory
supportive treatment at discharge. The results
were statistically homogeneous (P=0.29),
and a fixed effect model selected. Meta-analysis
showed that the final outcome of NIPPV was
significantly better than that of nCPAP as a
primary respiratory mode [OR=0.57 (95% CI:
0.37-0.88); P=0.01] (Web Fig. 2).
Three papers [17-19] reported
the comparison of NIPPV versus nCPAP in
the management of apnea of prematurity. The test
for heterogeneity was non-significant (P=0.21),
and a fixed effect model selected. Meta-analysis
showed a statistically lower rate of apnea
(episodes per hour) in the NIPPV group as
compared with nCPAP group [WMD=-0.48
(95%CI:-0.58-0.37; P<0.001] (Web
Fig. 3).
Five papers [13,16,20-21,25]
reported the comparison of duration of
hospitalization between NIPPV and nCPAP group
including the studies either as a primary
respiratory mode or as a extubation mode. A
random effect model was selected because of
significant heterogeneity (P=0.06).
Meta-analysis showed that there was no
significant difference in duration of
hospitalization between NIPPV and nCPAP group
[WMD=-0.51 (95%CI:-5.62-4.61; P=0.85] (Web
Fig. 4).
Table I showed the
incidence of BPD, IVH or PVL, ROP, pneumothorax
or air leak, abdominal distention, necrotizing
enterocolitis, and PDA in the group of NIPPV
versus nCPAP. Except for the incidence of
BPD (P=0.05), there was no significant
difference between the NIPPV and nCPAP groups
respectively (P>0.05).
TABLE I Meta-analysis of Secondary Outcomes between NIPPV and nCPAP Groups
|
|
n/N1) |
n/N2) |
Heterogeneity |
OR(95%CI) |
P |
|
BPD [13-14,16, 20-21,25] |
45/273 |
60/268 |
P=0.46 |
0.63 (0.39~1.00) |
0.05 |
|
IVH or PVL[14,17,20-21,25] |
36/219 |
46/216 |
P=0.76 |
0.70 (0.43~1.15) |
0.16 |
|
ROP [14,16,25] |
23/130 |
30/132 |
P=0.12 |
0.66 (0.35~1.25) |
0.20 |
|
pneumothorax or air leak[12,14,16,21,25] |
14/225 |
23/219 |
P=0.44 |
0.55 (0.27~1.10) |
0.09 |
|
abdominal distention[15,19] |
6/66 |
5/70 |
P=0.39 |
1.28 (0.37~4.44) |
0.70 |
|
necrotizing enterocolitis[14-16,21,25] |
12/227 |
19/224 |
P=0.71 |
0.61(0.29~1.28) |
0.19 |
|
PDA[14,16,25] |
50/166 |
51/161 |
P=0.67 |
0.92 (0.56~1.52) |
0.76 |
|
1): total patients of NIPPV group;
2): total patients of nCPAP group;
BPD: bronchopulmonary dysplasia; IVH:
intraventricular hemorrhage; PVL:
periventricular leukomalacia; ROP:
retinopathy; PDA: patent ductus
arteriosus |
Discussion
Respiratory distress syndrome
in preterm infants is still a big challenge for
neonatologists [26]. In recent year, increased
morbidity of NRDS in late-preterm and term
infants has been reported [27]. Although the
mortality of NRDS has been significantly reduced
the prolonged use of ETT and mechanical
ventilation might predispose the neonates to the
development of BPD. nCPAP has been widely used
as a non-invasive respiratory supportive mode
for NRDS [28]. However, nCPAP could not
consistently improve ventilation and could not
be effective in newborn infants with poor
respiratory effort. In fact, as many as 55%
preterm infants at the gestational age of 25-26
wk and 40% of 27-28 wk treated by nCPAP
developed respiratory failure and needed ETT and
mechanical ventilation within five days [29].
NIPPV has been suggested to have stronger
respiratory supportive effect than nCPAP [30].
NIPPV has been confirmed to decrease the work of
breathing in preterm infants with NRDS as
compared with nCPAP [31].
As compared with the
previously published meta-analyses [5-7,32]
on the comparison of NIPPV
and nCPAP, the present study also included the
newly published RCT articles, involved both
preterm and term infants, and assessed the
effect and safety in the round. The present
meta-analysis results showed that, as a primary
respiratory supportive mode, NIPPV could
significantly reduce the need for ETT and
mechanical ventilation, decrease the apnea
episodes of prematurity, and have a better
clinical outcome as compared with nCPAP. NIPPV
might be a valuable mode of primary respiratory
support. Till now, only one RCT study
investigated the comparison of NIPPV and
mechanical ventilation in preterm infants after
pulmonary surfactant administration [33], which
suggested that the group treated by NIPPV had
shorter duration of hospitalization, lower BPD
and mortality than that treated by mechanical
ventilation. A prospective observational study
also suggested that NIPPV was a safe and
effective primary mode of ventilation in
premature infants [34].
The present meta-analysis
results confirmed that NIPPV had a better effect
than nCPAP in the post-extubation period.
Moreover, NIPPV led to a marginally significant
reduction in the incidence of BPD as compared
with nCPAP. A clinical retrospective study also
suggested that NIPPV use in infants with birth
weight of 500-750 g was associated with
decreased BPD, BPD/death, and neurodevelopmental
impairment when compared with those managed with
nCPAP [35]. The present meta-analysis results
showed that there were no significant
differences in the incidence of IVH, PVL, ROP,
PDA, pneumothorax or air leak, abdominal
distention, necrotizing enterocolitis, and
duration of hospitalization between the group of
NIPPV and nCPAP. There were no other severe
complications associated with NIPPV or nCPAP
reported.
nCPAP has been confirmed to
be easy, and simple to use treatment of NRDS. As
compared with nCPAP, NIPPV might provide slight
but important beneficial effects. NIPPV has been
successfully established as an effective
treatment for NRDS, but the mechanism of action
of NIPPV needs further investigations. The
research on different ventilator equipment
(synchronized versus non-synchronized) or
method of synchronization should be continued.
Pressure variation during ventilator generated
NIPPV might have some negative effect in preterm
infants [36]. A randomized crossover trial of
four nasal respiratory support systems on apnea
of prematurity in very low birth weight infants
suggested that a variable flow nCPAP device
might be more effective than a conventional
ventilator in NIPPV mode [37]. NIPPV has been
provided by different study investigators using
different ventilator equipment (synchronized
versus non-synchronized) or method of
synchronization. Similarly, comparative nCPAP
has been provided using different types of
pressure generators. There is another new mode
of two-pressure level respiratory support
biphasic positive airway pressure (BiPAP). The
safety, i.e. long-term efficacy of these
different non-invasive respiratory supports need
further investigation [38].
Limitations of the present
meta-analysis: The study quality and
evidence validity was defined as moderate. Most
of the studies involved small number of patients
and therefore there was a wide confidence
interval in the pooled results. It’s difficult
to compare the sub-group of different
gestational age and birth-weight because of lack
of data for term infants. The effects of NIPPV
in late preterm and term neonates need further
studies. The present study had insufficient data
on important short term (IVH, PVL) and long term
(neurological) outcomes.
Contributors: ST
and JZ were responsible for data collection. JS
and ZH were responsible for computer-related
work. YS was responsible for writing the
submitted paper.
Funding: Clinical
Research Fund (2009) of Third Military Medical
University. Competing interests: None
stated.
|
What This Study Adds?
• NIPPV could
significantly reduce endotracheal
ventilation, increase successful
extubation, improve apnea of
prematurity, decrease the incidence of
BPD, and have better outcome as compared
with nCPAP.
|
References
1. DiBlarsi RM. Neonatal
noninvasive ventilation techniques: do we really
need to intubate? Respir Care. 2011;56:1273-97.
2. Bancalari E, Nelson C.
Non-invasive ventilation of the preterm infant.
Early Huma Develop. 2008;84:815-9.
3. Bhandari V. Nasal
intermittent positive pressure ventilation in
the newborn:review of literature and
evidence-based guidelines. J Perinatol.
2010;30:505-12.
4. Davis PG, Morley CJ, Owen
LS. Non-invasive respiratory support of preterm
neonates with respiratory distress: continuous
positive pressure and nasal intermittent
positive ventilation. Semin Fetal Neonatal Med.
2009;14:14-20.
5. Lampland AL, Meyers PA,
Worwa CT, Swanson EC, Mammel MC. Gas exchange
and lung inflammation using nasal intermittent
positive-pressure ventilation versus
synchronized intermittent mandatory ventilation
in piglets with saline lavage-induced lung
injury: an observational study. Crit Care Med.
2008;36:183–7.
6. Davis PG, Lemyre B, de
Paoli AG. Nasal intermittent positive pressure
ventilation (NIPPV) versus nasal continuous
positive airway pressure (NCPAP) for preterm
neonates after extubation. Cochrane Database
Syst Rev. 2001;3: CD003212.
7. Ho JJ, Subramaniam P,
Henderson-Smart DJ, Davis PG. Continuous
distending pressure for respiratory distress
syndrome in preterm infants. Cochrane Database
Syst Rev. 2002;2:CD002271.
8. Lemyre B, Davis PG, de
Paoli AG. Nasal intermittent positive pressure
ventilation (NIPPV) versus nasal
continuous positive airway pressure (NCPAP) for
apnea of prematurity. Cochrane Database Syst
Rev. 2002;1: CD002272.
9. Ma XL, Xu XF, Chen C, Yan
CY, Liu YM, Liu L, et al. Epidemiology of
respiratory distress and the illness severity in
late preterm or term infants: a prospective
multi-center study. Chin Med J (Eng).
2010;123:2776-80.
10. Kero PO, Makinen EO.
Comparison between clinical and radiological
classification of infants with respiratory
distress syndrome. Eur J Pediatr. 1979;130:
271-8.
11. Higgins JPT, Green S.
Cochrane Handbook for Systematic Reviews of
Interventions, Version 5.0.2.
12. Friedlich P, Lecart C,
Posen R, Ramicone E, Chan L, Ramanathan R. A
randomized trial of nasopharyngeal-synchronized
intermittent mandatory ventilation versus
nasopharyngeal continuous positive airway
pressure in very low birth weight infants after
extubation. J Perinatol. 1999;19:413-8.
13. Barrington KJ, Bull D,
Finer NN. Randomized trial of nasal synchronized
intermittent mandatory ventilation compared with
continuous positive airway pressure after
extubation of very low birth weight infants.
Pediatrics. 2001;107:638-41.
14. Khalaf MN, Brodsky N,
Hurley J, Bhandari V. A prospective randomized,
controlled trial comparing synchronized nasal
intermittent positive pressure ventilation
versus nasal continuous positive airway pressure
as modes of extubation. Pediatrics.
2001;108:13-7.
15. Khorana M, Paradeevisut
H, Sangtawesin V, Kanjanapatanakul W, Chotigeat
U, Ayutthaya JK. A randomized trial of
non-synchronized Nasopharyngeal Intermittent
Mandatory Ventilation (nsNIMV) vs. Nasal
Continuous Positive Airway Pressure (NCPAP) in
the prevention of extubation failure in pre-term
< 1,500 grams. J Med Assoc Thai.
2008;91:S136-42.
16. Moretti C, Giannini L,
Fassi C, Gizzi C, Papoff P, Colarizi P. Nasal
flow-synchronized intermittent positive pressure
ventilation to facilitate weaning in very low-birthweight
infants: unmasked randomized controlled trial.
Pediatr Int. 2008; 50:85-91.
17. Ryan CA, Finer NN, Peters
KL. Nasal intermittent positive-pressure
ventilation offers no advantages over nasal
continuous positive airway pressure in apnea of
prematurity. Am J Dis Child. 1989;143:1196-8.
18. Lin CH, Wang ST, Lin YJ,
Yeh TF. Efficacy of nasal intermittent positive
pressure ventilation in treating apnea of
prematurity. Pediatr Pulmonol. 1998;26:349-53.
19. Bisceglia M, Belcastro A,
Poerio V, Raimondi F, Mesuraca L, Crugliano C,
et al. A comparison of nasal intermittent
versus continuous positive pressure delivery for
the treatment of moderate respiratory syndrome
in preterm infants. Minerva Pediatr. 2007;
59:91-5.
20. Kugelman A, Feferkorn I,
Riskin A, Chistyakov I, Kaufman B, Bader D.
Nasal intermittent mandatory ventilation versus
nasal continuous positive airway pressure for
respiratory distress syndrome: a randomized,
controlled, prospective study. J Pediatr.
2007;150:521-6.
21. Kishore MSS, Dutta S,
Kumar P. Early nasal intermittent positive
pressure ventilation versus continuous
positive airway pressure for respiratory
distress syndrome. Acta Paediatr.
2009;98:1412-5.
22. Shi Y, Tang SF, Shen J,
Zhao JN, Hu ZX, Li HQ. Nasal intermittent
positive pressure ventilation versus
nasal continuous positive airway pressure for
the treatment of neonatal respiratory failure :
a prospective, randomized, controlled study.
Chin J Evid based Pediatr. 2009;4:494-8.
23. Shi Y, Tang SF, Zhao JN,
Hu ZX, Li TY. Efficiency of nasal intermittent
positive pressure ventilation vs nasal
continuous positive airway pressure on neonatal
respiratory distress syndrome: a prospective,
randomized, controlled study. Acta Acad Med Mil
Tertiae. 2010;32:1991-4.
24. Gao WW, Tan SZ, Chen YB,
Zhang Y, Wang Y. [Randomized trail of nasal
synchronized intermittent mandatory ventilation
compared with nasal continuous positive airway
pressure in preterm infants with respiratory
distress syndrome]. Chin J Contemp Pediatr.
2010;12:524-6.
25. Meneses J, Bhandari V,
Alves JG, Herrmann D. Noninvasive ventilation
for respiratory distress syndrome: A randomized
controlled trial. Pediatrics. 2011;127: 300-7.
26. Mathews TJ, MacDorman MF.
Infant mortality statistics from 2005 period
linked birth/infant death data set. Natl Vital
Stat Rep. 2008;57:1-32.
27. Hansen AK, Wisborg K,
Uldbjerg N, Henriksen TB. Elective caesarean
section and respiratory morbidity in the term
and near-term neonate. Acta Obstet Gynecol
Scand. 2007;86:389-94.
28. Dani C, Corsini I,
Bertini G, Fontanelli G, Pratesi S, Rubaltelli
FF. The INSURE method in preterm infants of less
than 30 weeks’ gestation. J Matern Fetal
Neonatal Med. 2010;23:1024-9.
29. Kirchner L, Weninger M,
Unterasinger L, Birnbacher R, Hayde M, Krepler
R, et al. Is the use of early nasal CPAP
associated with lower rates of chronic lung
disease and retinopathy of prematurity? Nine
years of experience with the Vermont Oxford
Neonatal Network. J Perinat Med. 2005;33:60-6.
30. Ramanathan R. Nasal
respiratory support through the nares: its time
has come. J Perinatol. 2010;30:S67-72.
31. Aghai ZH, Saslow JG,
Nakhla T, Milcarek B, Hart J, Lawrysh-Plunkett
R, et al. Synchronized nasal intermittent
positive pressure ventilation (SNIPPV) decreases
work of breathing (WOB) in premature infants
with respiratory distress syndrome (RDS)
compared to nasal continuous positive airway
pressure (NCPAP). Pediatr Pulmonol.
2006;41:875-81.
32. De Paoli AG, Davis PG,
Faber B, Morley CJ. Devices and pressure sources
for administration of nasal continuous positive
airway pressure (NCPAP) in preterm neonates.
Cochrane Database Syst Rev. 2008;1:CD002977.
33. Bhandari V, Gavino RG,
Nedrelow JH, Pallela P, Salvador A, Ehrenkranz
RA, et al. A randomized controlled trial
of synchronized nasal intermittent positive
pressure ventilation in RDS. J Perinatol.
2007;27:697-703.
34. Santin R, Brodsky N,
Bhandari V. A prospective observational pilot
study of synchronized nasal intermittent
positive pressure ventilation (SNIPPV) as a
primary mode of ventilation in infants > or = 28
weeks with respiratory distress syndrome (RDS).
J Perinatol. 2004;24:487-93.
35. Bhandari V, Finer NN,
Ehrenkranz RA, Saha S, Das A, Walsh MC, et al.
Synchronized nasal intermittent
positive-pressure ventilation and neonatal
outcomes. Pediatrics. 2009;124:517-26.
36. Owen LS, Morley, CJ,
Davis PG. Pressure variation during ventilator
generated nasal intermittent positive pressure
ventilation in preterm infants. Arch Dis Fetal
Neonatal Ed. 2010;95:F359-64.
37. Pantalitschka T, Sievers
J, Urschitz MS, Herberts T, Reher C, Poets CF.
Randomized crossover trial of four nasal
respiratory support systems on apnoea of
prematurity in very low birth weight infants.
Arch Dis Child Fetal Neonatal Ed. 2009;
94:F245-8.
38. Kieran EA, Walsh H,
O’Donnell CPF. Survey of nasal continuous
positive airways pressure (NCPAP) and nasal
intermittent positive pressure ventilation
(NIPPV) use in Irish newborn nurseries. Arch Dis
Child Fetal Neonatal Ed. 2011;96:F156.
|
|
|
 |
|

