|
Hepatoblastoma, the most common
malignant childhood liver tumor, has its highest incidence in the first
two years of life and shows a male preponderance. The overall 5-years
survival of this cancer had improved to 75% at the beginning of the 21st
century [1] compared to only 35% in the 1970s [2], by and large using
newer chemotherapy strategies [1-3] including a treatment strategy of
pre-operative chemotherapy with cisplatin and doxorubicin (PLADO)
followed by delayed surgical resection of the tumor.
There is a paucity of published information from
India on children with hepatoblastoma. This study aims to assess current
treatment outcomes of children with hepatoblastoma in India by a
comprehensive review of the published and grey literature.
Methods
A comprehensive search of Medline, Embase, Web of
Science and Scopus databases using keywords "hepatoblastoma" and "India"
was done. The search was limited to studies published from 2001 onwards.
Additionally, abstracts from annual Congresses of International Society
of Paediatric Oncology (SIOP) and American Society for Clinical Oncology
(ASCO) for the last 10 years (2001-2010) were hand-searched. Any single
or multi-center study from India was eligible for inclusion. If the
multi-center study from India was part of an international
collaboration, it was included if data specific to the Indian center was
available. If there was more than one study from the same institute,
only the study from the most recent time period was included. Case
reports were excluded. A data extraction form was created and
demographic, clinical and outcome data were extracted from the studies
identified. Outcomes of interest were survival, mortality, progression
of disease and abandonment of treatment.
Results
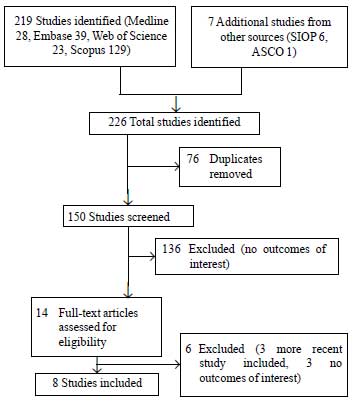
The initial search identified 226 studies (Fig.
1), of which eight studies (4 published and 4 abstracts from
scientific meetings) met the inclusion criteria for this review (Web
Table I) [4-11].
 |
|
Fig. 1 Flow diagram of study selection.
|
There were a total of 157 patients (range 5-36
patients in study) with a median age of 12 to 24 months. None of the
studies stratified patients for treatment based on stage or risk group.
In all the studies, majority of patients (range 67-100%) received
pre-operative chemotherapy mainly with PLADO followed by surgical
resection (75-100% of patients). The extent of resection was not always
specified. Surgery was followed by additional chemotherapy. The main
causes of treatment failure were progression of disease (range 0-30%)
and treatment related mortality (range 0-50%). The censored (excluding
those who abandoned treatment) event-free survival ranged from 33-100%
with varying degrees of follow-up. Although the data on treatment from
the four published studies were similar to the four abstracts from
scientific meetings, the outcomes of the published studies were
generally better. Only three studies reported treatment abandonment that
ranged from 25% to 62% [6,10,13].
Discussion
Before drawing conclusions based on the observations
in the studies identified in this review, certain caveats need to be
considered. Firstly, only a small number of studies have been
identified, all of which are single-center, mostly retrospective
case-series and some have not been published (hence not peer-reviewed).
Secondly, key baseline information like stage of disease at presentation
was either not specified or specified by using the American (POG) or
European (PRETEXT) classification system, thus limiting an understanding
of factors that affect outcomes. Finally, there was variable inclusion
and reporting of outcomes on those who refused or abandoned treatment.
To allow for comparability, the survival data is presented (Web
Table I) after censoring those who abandoned treatment. Despite
these caveats, this review allows us to make several important
observations.
The treatment-related mortality was mainly due to
sepsis, but there were also some peri-operative deaths. There were no
deaths due to cardiotoxicity although not all studies gave details of
toxicity. In the treatment of hepatoblastoma, myelosuppression caused by
chemotherapy and contributing to sepsis can be significant and these
toxicity-related deaths may reflect challenges in providing optimal
supportive care. Using cisplatin alone for treating standard risk
hepatoblastoma (which has been shown to be as effective as PLADO [12])
in a resource-limited setting like India has clear advantages. This
would make the treatment less myelosuppressive, potentially leading to a
decrease in treatment related morbidity and mortality. Additionally, the
treatment is likely to be more cost-effective, which can have a knock-on
effect on reducing treatment abandonment [13]. A multi-center pilot
study [14] in India is currently underway to study the efficacy and
affordability of cisplatin monotherapy and this should provide some
answers to the above hypotheses.
The other reason for treatment failure was
progression of disease. It is likely that a greater proportion of those
who progressed had high-risk hepatoblastoma at presentation although
there was insufficient data in the individual studies to confirm this.
Patients with high-risk hepatoblastoma need more intense initial
chemotherapy which has to be followed-up by complete hepatectomy and
subsequent orthotopic liver transplantation in a proportion of patients
[15]. At present, this treatment strategy is not prevalent in India
although the first successful liver transplant for hepatoblastoma has
been recently reported from the country [16]. While such a strategy of
more intense chemotherapy along with better supportive care could
improve outcomes, the gains are likely to be modest in the absence of
widespread availability of liver transplantation as a therapeutic
option.
In addition to treatment and supportive care related
factors, stage of disease at presentation has been consistently shown to
be related to prognosis [17]. In India, one might anticipate that as a
consequence of economic and healthcare infrastructure barriers, there
may be delays in presentation that could lead to an advanced stage at
presentation and consequently an adverse outcome. From this review,
there is little evidence of this promise. Five of the eight studies
[6,7,9-11] reported stage of disease at presentation and in all except
one [7], this was not different from that reported from resource-rich
nations [1-3]. Future larger multicentre studies from India need to
answer this question in a more definitive way.
Acknowledgment: Dr Barry Pizer for his
very useful comments about the manuscript.
Contributors: RSA conceived the idea, searched
the literature and prepared the manuscript. He will act as guarantor of
the paper.
Funding: None; Competing interests: None
stated.
|
What This Study Adds?
• Survival rates for children with
hepatoblastoma in India range from 33-100%, with
toxicity-related deaths and progression of disease being the
main causes of treatment failure.
|
References
1. Pritchard J, Brown J, Shafford E, Perilongo G,
Brock P, Dicks-Mireaux C, et al. Cisplatin, doxorubicin, and
delayed surgery for childhood hepatoblastoma: a successful
approach–results of the first prospective study of the International
Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819-28.
2. Exelby PR, Filler RM, Grosfeld JL. Liver tumors in
children in the particular reference to hepatoblastoma and
hepatocellular carcinoma: American Academy of Pediatrics Surgical
Section Survey–1974. J Pediatr Surg. 1975;10:329-37.
3. Ortega JA, Douglass EC, Feusner JH, Reynolds M,
Quinn JJ, Finegold MJ, et al. Randomized comparison of cisplatin/vincristine/fluorouracil
and cisplatin/continuous infusion doxorubicin for treatment of pediatric
hepatoblastoma: A report from the Children’s Cancer Group and the
Pediatric Oncology Group. J Clin Oncol. 2000;18:2665-75.
4. Shah PM, Shah R, Shah N. Heptoblastoma – GCRI
experience [abstract]. Med Pediatr Oncol. 2001;37:288.
5. Udupa KV, Navadgi SM, Mullerpatan P, Chhabra D,
Shah RC, Jagannath P. Neoadjuvant chemotherapy before surgery of
hepatoblastoma. Indian J Pediatr. 2006;73: 735-7.
6. Agarwala S, Bakshi S, Bajpai M, Bhatnagar V, Gupta
AK, SD Gupta, et al. Validation of PRETEXT staging system and
risk categorization for prognostication and outcome in hepatoblastoma –
results from AIIMS-HB94 trial [abstract]. Pediatr Blood Cancer.
2007;49:401-2.
7. Mehta P, Buch N, Swami A, Bhatnagar S, Shah N,
Desai M, et al. Difficulties faced during treatment of
hepatoblastoma – an Indian experience from a tertiary charitable trust
hospital [abstract]. Pediatr Blood Cancer. 2007;49:535-6.
8. Thankamony P, Raghavan H, Kattoor J, Parukutty K.
Hepatoblastoma – ten year experience from Regional Cancer Centre,
Thiruvanathapuram, Kerala, South India [abstract]. Pediatr Blood Cancer.
2007;49:535.
9. Shukla PJ, Barreto SG, Qureshi SS, Hawaldar R,
Shrikhande SV, Ramadwar MR, et al. Hepatoblastoma: a single
institutional experience of 18 cases. Pediatr Surg Int. 2008;24:799-802.
10. Singh T, Satheesh CT, Appaji L, Aruna Kumari BS,
Padma M, Kumar MV, et al. Hepatoblastoma: Experience from a
single center. Indian J Cancer. 2010;47:314-6.
11. Cyriac S, Seshadri RA, Warrier A, Sagar TG.
Hepatoblastoma: Analysis of treatment outcome from a tertiary care
center. J Indian Assoc Pediatr Surg. 2011;16:11-4.
12. Perilongo G, Maibach R, Shafford E, Brugieres L,
Brock P, Morland B, et al. Cisplatin versus cisplatin plus
doxorubicin for standard-risk hepatoblastoma. N Engl J Med.
2009;361:1662-70.
13. Arora RS, Pizer B, Eden TO. Understanding refusal
and abandonment in the treatment of childhood cancer. Indian Pediatr.
2010;47:1005-10.
14. Agarwala S, Ronghe MD, Aronson DC, Czauderna P,
Brock P, Roebuck D, et al. Pilot of treatment guidelines for
hepatoblastoma (HB) in resource challenged nations (RCN) [abstract].
Pediatr Blood Cancer. 2009;53:717.
15. Zsíros J, Maibach R, Shafford E, Brugieres L,
Brock P, Czauderna P, et al. Successful treatment of childhood
high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and
surgery: final results of the SIOPEL-3HR study. J Clin Oncol.
2010;28:2584-90.
16. Rao S, D’Cruz AL, Aggarwal R, Chandrashekar S,
Chetan G, Gopalakrishnan G, et al. Pediatric liver
transplantation: A report from a pediatric surgical unit. J Indian Assoc
Pediatr Surg. 2011;16:2-7.
17. Shafford EA, Pritchard J. Liver tumours. In: Pinkerton R,
Plowman PN, Pieters R, editors. Paediatric Oncology. 3rd ed.
London: Arnold; 2004. p. 448-68.
|

