|
|
|
Indian Pediatr 2012;49: 277 -280 |
 |
Adiponectin as a Marker of Complications in
Type I Diabetes
|
|
Nevin Mohamed Mamdouh Habeeb, Omneya Ibrahim Youssef,
*Azza Abdel Rahman Saab
and *Eman Saleh El Hadidi
From the Department of Pediatrics and *Clinical
Pathology, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
Correspondence to: Omneya Ibrahim Youssef, Lecturer of
Pediatrics, Faculty of Medicine, Ain Shams University. 29Dar EL Ez,
Medinet El Zahraa, Helmeyet El Zaytoun, Cairo, Egypt.
Email: [email protected]
Received: November 09, 2010;
Initial review: January 5, 2011;
Accepted: May 19, 2011;
Published online: 2011 August 15.
PII: S09747559INPE1000420-1
|
Objective: To evaluate adiponectin levels in children and
adolescents with type I diabetes, and their relationship to long term
complications.
Design: Cross sectional.
Setting: Tertiary referral hospital, Cairo,
Egypt.
Participants: Thirty children and adolescents
with type I diabetes mellitus, classified into complicated and
non-complicated and compared to 10 healthy age and sex matched subjects
as a control group.
Methods: All children underwent anthropometric
measurements, neurological assessment, fundus examination,
echocardiography and assays of HbA1c, creatinine, 24-hr urinary protein,
and serum adiponectin.
Main outcome measure: Relationship of serum
adiponectin to complications of type I diabetes mellitus, and glucose
control.
Results: Serum adiponectin was
significantly elevated in complicated diabetes (10.3±5.9 pg/dL) as
compared to the controls (6.5±3.7pg/dL) (P<0.01), and correlated
directly with HbA1c (P<0.05) and creatinine (P<0.001).
Patients with nephropathy showed high values of adiponectin (15.7±3.7
pg/dL).
Conclusion: Elevated adiponectin level in
children and adolescents with type I diabetes indicates poor glycemic
control and development of complications, especially nephropathy.
Key words: Adiponectin, Complications, Diabetes, Prognosis.
|
|
Type I diabetes mellitus is characterized by
marked inability of the pancreas to secrete
insulin [1]. The morbidity and mortality
associated with diabetes is related to its short and long term
complications [2]. Adiponectin is a protein hormone that
modulates a number of metabolic processes. Levels of the hormone are
inversely correlated with body mass index (BMI) [3]. In adults,
lower circulating levels of the adipocyte-derived hormone are associated
with obesity, type 2 diabetes and microvascular disease risks. In type I
diabetic patients, the relationship between adiponectin and the presence
of vascular complications is largely unknown [4]. Further, its
use as a risk marker in children is less clear [5].
We conducted this study to evaluate the levels of
adiponectin in children and adolescents with type I diabetes, and its
possible relationship to the occurrence of complications.
Methods
This cross sectional study, comprised 30 children and
adolescents recruited consecutively from Children’s hospital, Ain Shams
University. They were diagnosed with type I diabetes according to the
American Diabetes Association criteria [6]. Based on the results
of the evaluation, they were classified into complicated group (with one
or more of vascular complications namely retinopathy, nephropathy and
cardiomyopathy) and non complicated group. Ten age and sex matched
healthy children and adolescents were studied as a control group. After
obtaining an informed consent all subjects enrolled in the study were
subjected to: history taking, thorough clinical examination stressing on
anthropometric measurements to calculate the body mass index (BMI) (data
were plotted on sex and age specific charts to determine whether each
subject is below or above the 85th percentile [7]),sex maturity
rating to obtain the Tanner score [8], neurological examination,
as well as fundus examination. Echocardiographic evaluation was
performed using Vivid 7 Dimension, GE (Vingmed ultrasound AS N-3190
Horten, Norway), left ventricular (LV) systolic function was determined
by estimation of ejection fraction (EF), LV diastolic function was
determined through estimation of peak flow rate of e wave, a wave and
(e/a) [9].
Laboratory investigations comprised measure-ment of
serum creatinine, urinary microalbumin assay (immuno-turbididmentric
method), glycated hemoglobin level (HbA1c) and adiponectin assay (R&D
systems, Inc 614 McKisley place, N.E. Minneapolis, MN 55413, USA). This
assay employs the quantitative sandwich enzyme immunoassay technique
performed in microplates. Data were analyzed using SPSS.
This study was approved by the Ethical Committee of
the Pediatric Department, Ain Shams University.
Results
Mean age of subjects in complicated and uncomplicated
group was 16.1±2.8 y, and 14.7±2.9 y, respectively. Age of control group
was 13.1±2.9 y. The mean duration of illness of complicated diabetics
was 10.4±2.2 y, their glycated Hb was significantly elevated
(10.30±1.98%). Their Tanner score ranged from 1-5 with a mean of
3.73±1.08, six (40%) of them had body mass index (BMI) >85th percentile.
Neuropathy occurred in 9 patients, retinopathy in 8,
cardiomyopathy in one patient (ejection fraction 43%, e/a ratio 0.8),
and one patient displayed only diastolic dysfunction with e/a ratio of
0.75. The mean ejection fraction in the whole group was (63±5.67%) and
the mean e/a ratio was (1.6±1.37). Elevated creatinine (4.23±2.03mg/dL)
was found in 10 patients, all of them had albuminuria >30mg/dL and their
blood pressure was controlled on ACE inhibitors. Adiponectin level was
significantly elevated in complicated patients (10.3±5.9 pg/mL) in
comparison to the level in uncomplicated patients (5.04±4.3 pg/mL) as
well as the control group (6.5±3.7 pg/mL) (P<0.001 and P>0.1,
respectively). Comparison between patients with and patients without
complications as regards the levels of glycated Hb and adiponectin are
presented in Table I. The single patient with
established cardiomyopathy had retinopathy as well; his adiponectin was
12.5pg/dL.
TABLE I Duration of Disease, Adiponectin Level and Hba1c in Complicated and Non-complicated Diabetics
|
Variables |
Absent |
Present |
|
Retinopathy |
|
Adiponectin (pg/dL) |
9.7+4.6 |
10.9+7.1 |
|
*HbA1c % |
9.2+0.6 |
11.3+1.9 |
|
Duration of DM (y) |
11+1.7 |
10+2.7 |
|
Neuropathy |
|
Adiponectin (pg/dL) |
11.3+6.1 |
9.7+6 |
|
HbA1c % |
10.4+1.9 |
10.2+1.8 |
|
Duration of DM (y) |
10+1.8 |
10.2+2.6 |
|
Nephropathy |
|
#Adiponectin (pg/dL) |
6.8+4.2 |
15.7+3.7 |
|
*HbA1c % |
9.6+1.5 |
11.3+1.7 |
|
Duration of DM (y) |
10.8+1.9 |
10+2.9 |
|
*P<0.05; #P<0.001; DM= diabetes mellitus, Y=year, Hb A1c = glycated hemoglobin. |
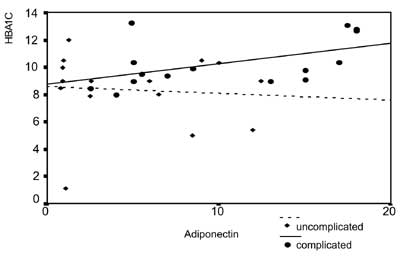 |
|
Fig. 1 Correlation between adiponectin
and glycated hemoglobin in children with uncomplicated and
complicated diabetes mellitus.
|
Adiponectin was directly correlated with HbA 1c
(Fig. 1), and serum creatinine (P<0.001)
and inversely correlated with the Tanner score (P<0.05). Within
the complicated group; comparisons between those with BMI> and <85th
percentile, high and low Tanner score, and females and males as regard
the adiponectin level are presented in Table II.
Table II Association of Adiponectin Levels With Bmi, Sexual Maturity and Gender in Diabetic Children
|
Variables
|
Complicated |
Uncompleted |
|
group |
group
|
|
Body Mass Index* |
|
< 85th percentile |
13.7±5.2 |
7±4 |
|
>85th percentile |
5.4±2.1 |
1±0.2 |
|
Tanner score# |
|
4-5 |
6.5±4.4 |
1±0.2 |
|
<4 |
12.9±5.4 |
7.7±3.6 |
|
Gender
|
|
Female |
5.9±4.8 |
12.3±5.3 |
|
Males |
8.8±6.2 |
2.3±2.4 |
|
*P<0.001 for comparison in both complicated and
uncomplicated group; P<0.001 in complicated group and <0.005 in
uncomplicated group. |
In the non complicated group the mean glycated Hb
(7.80±1.73%) was significantly lower than in complicated patients (P<0.05).
The mean duration of their illness was (6.5±3.5y). Their Tanner score
ranged from 1-5 with a mean of 3.1±1.3, five of them had a BMI >85th
percentile.
Discussion
The current study showed that the adipocyte derived
cytokine adiponectin was significantly high in poorly controlled
diabetics with high glycated Hb. Celi, et al. [10] reported
similar findings. The collagenous domain of the adiponectin molecule has
four conserved lysines. Glycosylation of these molecules is one of the
major post-translation modifications of adiponectin. In diabetic
patients with constant hyperglycemia, the glycosylation process is
altered, and this could lead to an altered adiponectin function.
Consequently, a modified adiponectin molecule could lead to diminished
negative feedback, and thus to increased adiponectin concentrations in
diabetics [11].
Among the complicated group, adiponectin level was
strikingly elevated in patients with nephropathy. Saraheimo, et al.
[12], elucidated a relationship between adiponectin and nephropathy.
Renal insufficiency per se could stimulate adiponectin production
or alternatively lead to a defect in the clearance of adiponectin. The
latter suggestion is supported by the finding that successful kidney
transplantation is followed by decreased adiponectin concentration
[13]. Adiponectin itself may have a role in mitigating the
mircrovascular and macrovascular burden in diabetic nephropathy.
Treatment with angiotensin converting enzyme inhibitor (ACEI) was also
associated with an increase in adiponectin level [14].
Adiponectin levels were also elevated in patients
with retinopathy, neuropathy and in the single patient with
cardiomyopathy. Hadjadj, et al. [15] reported that elevated
adiponectin observed in subjects with mircrovascular and macrovascular
diseases may indicated an altered regulation of this adipocytokine in
patients with complications associated with type I diabetes.
Adiponectin level was normal in the studied
uncomplicated diabetic patients, as also observed earlier [3,16].
Chronic exposure to insulin (as in type 2 diabetes) decreases the gene
expression of adiponectin in cultured adipocytes, suggesting that
absolute insulin deficiency may contribute to elevated level of serum
adiponectin in type I diabetes, but appropriate regular treatment with
insulin returned these levels to normal [17].
We conclude that adiponectin levels are high in
complicated type I diabetic children and adolescents especially those
who developed nephropathy, and it can reflect poor glycemic control.
Meanwhile, it remained normal in uncomplicated diabetics. BMI and
pubertal development exert negative effect on circulating adiponectin.
Contributors: All authors contributed to the
study design and drafting of manuscript.
Funding: None; Competing interests: None
stated.
|
What is Already known?
• Adiponectin levels decrease in type 2
diabetes but increases in type 1 diabetes in the presence of
complications.
What This Study Adds?
• Levels of rise of adiponectin differs by
the type of complications, and are also affected by puberty and
BMI in type I diabetic children with complications.
|
References
1. Votey SR, Peters AL. Diabetes Mellitus,
Type 1-A Review-emergency medicine. Available at:
http://emedicine.medscape.com/article/766036-overview. Accessed on
January 15, 2010.
2. CDC: Centers for Disease Control and Prevention.
National Diabetes Fact Sheet. United States. 2003. Available from:
http://www.cdc.gov/diabetes/pubs/pdf/ndfs. Accessed on February 15,
2010.
3. Morales A, Wasserfall C, Brusko T, Carter
C, Schatz D, Silverstein J, et al. Adiponectin and leptin
concentrations may aid in discriminating disease forms in children and
adolescents with type 1 and type 2 diabetes. Diabetes Care. 2004;27:
2010-4.
4. Frystyk J, Tarnow L, Hansen TK, Parving HH,
Flyvbjerg A. Increased serum adiponectin levels in type 1
diabetic patients with mircrovascular complications. Diabetologia. 2005;
48:1911-8.
5. Ong KK, Frystyk J, Flyvbjerg A, Petry CJ, Ness A,
Dunger DB. Sex-discordant associations with adiponectin levels
and lipid profiles in children. Diabetes. 2006;55:1337-41.
6. Expert Committee on the Diagnosis and
Classification of Diabetes Mellitus. Report of the Expert
Committee on the Diagnosis and Classification of Diabetes Mellitus.
Diabetes Care. 26 (Suppl. 1). 2003:S5-20.
7. Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding
A, Goran MI, Dietz WH. Validity of body mass index compared with
other body-composition screening indexes for the assessment of body
fatness in children and adolescents. Am J Clin Nutr. 2002;75:978-85.
8. Marshall WA, Tanner JM. Variations in the pattern
of pubertal change in boys. Arch Dis Child. 1970; 45:13-23.
9. Sahn A. Determination of pulmonary to systemic
blood flow ratio in children by simplified doppler echocardiographic
method. J Am Coll Cardiol. 1989;1:825-30.
10. Celi F, Bini V, Papi F, Santilli E,
Castellani MS, Ferretti A, et al. Circulating
adipocytokines in non-diabetic and Type 1 diabetic children:
relationship to insulin therapy, glycaemic control and pubertal
development. Diabet Med. 2006;23:660-5.
11. Wang Y, Xu A, Knoght C, Xu L, Cooper G.
Hydroxylation and glycosylation of the four conserved lysine residues in
the collagenous domain of adiponectin. J Biol Chem. 2002;277:19521-9.
12. Saraheimo M, Forsblom C, Fagerudd J. Serum
adiponectin is increased in type 1 diabetic patients with nephropathy.
Diabetes Care. 2005;28:1410-4.
13. Chudek J, Adamezak M, Karkoszka H. Plasma
adiponectin concentration before and after successful kidney
transplantation. Trans Proc. 2003;35:2186-9.
14. Zoccali C, Mallamaci F, Tripepi G, Benedetto FA,
Cutrupi S, Parlongo S, et al. Adiponectin metabolic risk factors
and cardiovascular events among patients with end-stage renal disease. J
Am Soc Nephrol. 2002;13:134-41.
15. Hadjadj S, Aubert R, Fumeron F, Pean F, Tichet J,
Roussel R, et al. Increased plasma adiponectin concentrations are
associated with mircoangiopathy in type 1 diabetic subjects.
Diabetologia. 2005;48:1088-92.
16. Imagawa A, Funahashi T, Nakamura T. Elevated
serum concentration of adipose-derived factor, adiponectin, in patients
with type 1 diabetes. Diabetes Care. 2002;25:1665-6.
17. Fasshauer M, Klein J, Neumann S, Eszlinger M,
Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1
adipocytes. Biochem Biophys Res Commun. 2002;290:1084-9.
18. Punthakee Z, Delvin, Otoughlin J. Adiponectin,
adiposity, and insulin resistance in children and adolescents. J Clin
Endorcinol Metab. 2006; 91:2119-25.
19. Tsou P, Jiang Y, Chang CC. Sex-Related differences between
adiponectin and insulin resistance in school children. Diabetes Care.
2004;27:308-13.
|
|
|
 |
|

