|
|
|
Indian Pediatr 2009;46: 310-338 |
 |
Drug Therapy of Cardiac Diseases in Children |
|
Working Group on Management of Congenital Heart Diseases in India
Correspondence to: Dr Anita Saxena, Professor of
Cardiology, All India Institute of Medical Sciences,
New Delhi 110029, India.
E mail: anitasaxena@hotmail.com
|
|
Abstract
Justification: The indications and doses of most
drugs used for heart ailments in children are extrapolated from data in
adult patients. Separate guidelines are needed for neonates, infants and
children because of the differences in underlying heart diseases and
metabolic clearance of some of these drugs.
Process: Consensus emerged following expert
deliberations at the National Meeting on Management of Congenital Heart
Diseases in India, held on 13th September 2008, at the All India
Institute of Medical Sciences, New Delhi, India, supported by Pediatric
Cardiac Society of India.
Objectives: To review the literature and frame
evidence based guidelines for (i) indications, doses, adverse
effects and safety profile of commonly used drugs in pediatric
cardiology practice; and (ii) to provide an algorithm for
treatment in various clinical settings.
Recommendations: Consensus review and
recommendations are given for drugs used in children for heart failure,
hypertension, thrombosis, supraventricular tachycardia and intensive
care. Guidelines are also given for use of intravenous immunoglobulins
and sildenafil in children.
Key words: Children, Drug doses, Drug therapy, India,
Pediatric cardiology.
|
|
The list of drugs used for various cardiac
diseases in children is long and ever increasing. Most of the data for
efficacy of these drugs has been generated in adult cardiac patients
through randomized trials and observational studies. Conducting such
trials in children is difficult, if not impossible, due to logistic
problems and ethical issues. Therefore, in most cases the basis of using a
drug in pediatric practice is extrapolated from the experience in adult
patients.
With this background, the Working Group on Management
of Congenital Heart Diseases met on 13th September 2008, at the All India
Institute of Medical Sciences. New Delhi, to reach a consensus for
evidence based review of drugs used in heart disease in children and
formulation of recommendations.
The recommendations are classified into three
categories according to their strength of agreement:
Class I: General agreement exists that the
treatment is useful and effective.
Class II: Conflicting evidence or divergence of
opinion or both about usefulness/efficacy of treatment.
IIa: Weight of evidence/opinion is in favor of
usefulness/efficacy.
IIb: Usefulness/efficacy is less well
established.
Class III: Evidence and/or general agreement that
the treatment is not useful and in some cases may be harmful.
The following review is based on the presentations and
discussion of the Working Group. A consensus was reached to provide
recommendations for drug therapy under the sub-headings of various
clinical settings. The pharmacokinetics and pharmacodynamics of individual
drugs will only be briefly mentioned, as and when necessary.
Heart Failure
Heart failure is a clinical syndrome characterized by
the inability of the heart to supply cardiac output at a pace necessary to
meet the metabolic demands of the body. In children, the requirement also
includes "growth and development". Heart failure in children may occur
secondary to congenital or rheumatic heart disease. The causes of heart
failure in children are summarized in Table I.
Table I
Causes of Heart Failure
|
Volume overload with preserved systolic ventricular function
|
|
Large left to right shunt: VSD, AVSD, PDA |
|
Admixture lesions with high PBF: TGA,
TAPVC, Truncus |
|
Regurgitant lesions: MR, AR
(Rheumatic/Congenital) |
|
Myocyte dysfunction with abnormal ventricular contractile function |
|
Pressure overload: Severe AS, PS |
|
Muscular dystrophy, DCM |
|
Inflammatory: Myocarditis, Chaga’s, HIV |
|
Tachycardiomyopathies secondary to SVT |
|
Abnormal morphology: single ventricle (pre
and post op) |
|
Ischemic: ALCAPA |
|
Others: Sepsis, post CPB, hypocalcemia
etc. |
ALCAPA: anomalous left coronary artery from pulmonary artery, AR: aortic regurgitation, AS: aortic stenosis,
AVSD: atrioventricular septal defect, CPB: cardiopulmonary bypass, DCM: dilated cardiomyopathy,
HIV: human immunodeficiency virus, MR: mitral regurgitation, PBF: pulmonary blood flow,
PDA: patent ductus arteriosus, PS: pulmonary stenosis, SVT: supraventricular tachycardia,
TAPVC: total anomalous pulmonary venous connection, TGA: transposition of great arteries,
VSD: ventricular septal defect.
|
A. Drugs Used For Treatment of Heart Failure
1. Digoxin
Digoxin is a digitalis glycoside. It inhibits the
sodium potassium adenosine triphosphatase (Na-K-ATPase), increasing the
intracellular calcium levels, thereby increasing the contractile state of
the myocardium. Inhibition of Na-K-ATPase also reduces sympathetic flow
from the central nervous system and reduces the renal absorption of sodium
in the kidney(1). This leads to suppression of renin secretion from the
kidneys(2). Digoxin increases the vagal tone, thereby increasing the
refractory period and slowing the conduction through the sinus node and
the atrioventricular node. Digoxin is the only oral inotropic drug.
Indications: Digoxin is indicated in heart
failure associated with reduced systolic function of heart. In most cases
of heart failure, digoxin is combined with a diuretic and an angiotensin
converting enzyme inhibitor (ACEi). Its role in heart failure secondary to
left to right shunt lesions, where systolic function of the myocardium is
preserved, is not well defined. Digoxin is used for slowing ventricular
rate in tachyarrhythmias such as supraventricular tachycardia (SVT),
atrial flutter and atrial fibrillation (AF).
Evidence: Digoxin is shown to improve symptoms in
patients with heart failure(3). However, it has not been shown to provide
survival benefit in adults or in children(4). Lower dose may reduce the
incidence of side effects and toxicity(DIG trial)(5). In a post hoc
analysis of DIG trial, higher serum digoxin levels were associated with
increased mortality in men with heart failure(6). Scant data exist for
digoxin therapy in children with heart failure. Utility of digoxin in
heart failure secondary to volume overload of the ventricle, as seen in
left to right shunt lesions, is less clear, since the myocardial
contractility is normal in such cases(7).
Dosage: See (Table II). Rapid
digitalization is usually not indicated when using digoxin for heart
failure(9). Rapid digitalization may be indicated for treatment of acute
tachyarrhythmias. The maintenance dose is given in twice daily doses for
children under 10 years and once daily for children above 10 years.
Digoxin "holiday" is generally not needed in children. The half life of
digoxin is markedly prolonged in preterm babies and in those with renal
dysfunction. Dose of digoxin should he halved when using amiadarone.
Table II
Dosing for Digoxin in Infants and Children(8)
|
Age |
Total digitalizing
dose mcg/kg/24 hr |
Daily maintenance dose mcg/kg/24 hr |
| |
PO |
IV |
PO |
IV |
|
Premature newborn |
20 |
15 |
5 |
3-4 |
|
Full term newborn |
30 |
20 |
8-10 |
6-8 |
|
<2 yr |
40-50 |
30-40 |
10-12 |
7.5-9 |
|
2-10 yr |
30-40 |
20-30 |
8-10 |
6-8 |
|
>10 yrs |
0.75-1.5 mg |
0.5-1 mg |
0.125-0.5mg |
0.1-0.4mg |
PO: per oral; IV: intravenous.
|
Side effects: Digoxin has a narrow therapeutic
range and side effects are not uncommon. Heart blocks are more common in
children; ectopy is more often seen in adults. Side effects include:
• Cardiac arrhythmias – Sinus bradycardia, sinoatrial
and atrioventricular blocks, atrial and nodal ectopic beats, atrial
tachycardia with block, ventricular arrhythmias including ventricular
tachycardia (VT).
• Gastrointestinal – nausea, vomiting, abdominal pain
and diarrhea.
• Central nervous system – lethargy, confusion,
disorientation, vertigo, headache, fatigue, anxiety, depression,
delirium, and hallucinations
• Endocrine and Metabolic – Hyperkalemia with acute
toxicity.
• Ocular – blurred vision, haloes, yellow/green
vision, diplopia, photophobia, flashing lights.
Contraindications:
Absolute: Hypersensitivity to digoxin, ventricular
fibrillation, sick sinus syndrome, atrioventricular blocks and
hypertrophic obstructive cardio-myopathy.
Relative: Hypoxia, hypothyroidism, acute
myo-carditis, pre-excitation like WPW syndrome (if >2 years of age),
electrolyte disorders and acute myocardial infarction.
The dose of digoxin should be reduced in renal
impairment. Concomitant use of calcium channel blockers should be avoided.
Monitoring: Heart rate and rhythm should be
monitored. Periodic ECGs are recommended when uptitrating the dose or
using diuretics. Serum calcium, potassium and renal parameters need to be
monitored. If suspecting toxicity, serum digoxin levels should be measured
(sample taken at least 6 hours after the dose). Toxicity is usually seen
at >2 ng/mL level.
Preparations: Digoxin is available as elixir
(60 mL suspension, 50ug/mL) and tablet (0.25 mg tab). Tablets should not
be crushed to formulate liquid preparations for children. Injectable
digoxin (100 µg/mL, 250 µg/mL) is available for intravenous use.
Intramuscular route is not recommended.
2. Diuretics
Diuretics are widely used in heart failure because of
the symptomatic relief from fluid overload with in minutes of
administration. Diuretics are currently recommended for all adult patients
with heart failure who have volume overload of the ventricle(10). These
drugs can be classified into three categories, according to their site of
action on the kidney. Diuretics from different groups can be combined for
greater efficacy:
(a) Loop diuretics: Act on the
ascending limb of loop of Henle, resulting in Na, K+,
chloride and water excretion. Examples include Furosemide, torsemide.
(b) Thiazides: These drugs act at the
distal convoluted tubule and also result in Na, K+
and chloride excretion. Examples include hydro-chlorothiazide and
metolazone.
(c) Aldosterone antagonists: These
drugs act primarily by competing for intracellular aldosterone receptors
in the distal tubule. The excretion of water and Na is increased, while
K+ excretion is spared. Examples
include spironolactone and eplerenone
Furosemide
Furosemide is a loop diuretic and is a preferred agent
in heart failure due to its rapid onset of action, high efficacy with
greater fluid clearance. Increasing doses have increasing efficacy and it
remains effective even at low glomerular infilteration rate (GFR).
Furosemide is three times more potent than thiazide diuretics. Furosemide
also has a venodilatory effect and increases systemic venous capacitance,
thereby reducing preload.
Indications: Furosemide is indicated in heart
failure, pulmonary edema, hypertension, renal failure, and for fluid
overload due to other causes. When using diuretic, one must make sure that
there is no hypovolemia (as may be seen in postoperative settings and in
newborns)
Evidence: Furosemide is proven to be beneficial for
symptomatic relief. No survival benefit has been shown for patients with
heart failure.
Dosages and Pharmacodynamics: Oral: 1-2
mg/kg every 12 hours, maximum of 4 mg/kg/day; intravenous: 1 mg/kg/dose up
to 3-4 times a day; Continuous IV infusion: 1-4 mg/kg/day. Continuous
infusion may be better and safer in acute heart failure and in
postoperative setting. The onset of action starts in 10-20 minutes after
an IV dose and 20-30 minutes after oral administration. The duration of
action is six hours.
The dose does not need to be adjusted in renal or
hepatic impairment. Furosemide may increase chances of digoxin toxicity by
producing hypo-kalemia. It activates the renin angiotensin aldosterone
axis (RAAS), producing vasoconstriction, which is detrimental in heart
failure. Concomitant use of ACEi (vasodilator) is recommended, whenever
possible.
Preparations: Furosemide is available as 40 mg
tablet and 10 mg/mL (2 mL amp) injections. The cost is quite low. Oral
liquid preparation is not available in Indian market.
Side effects: These are dose dependent and include
the following:
1. Electrolyte imbalance: Most common and most
dreaded side effects.
(a) Hyponatremia: hyponatremia is
common, especially with high doses and it results in further drug
resistance. It should be managed with fluid restriction.
(b) Hypokalemia: Hypokalemia may
increase chances of digoxin toxicity. It is best managed by combining
furosemide with ACEi or spironolactone. Alternatively, potassium
supplements may be prescribed.
(c) Chloride depletion, leading to metabolic
alkalosis. Supplementation with potassium chloride helps.
2. Metabolic alkalosis, hyperuricemia.
3. Impaired glulcose tolerance leading to
hyperglycemia.
4. Increased low density lipoprotein cholesterol and
triglycerides.
5. Ototoxicity: Rapid administration of large doses
may cause ototoxicity, rare in children. When furosemide is used along
with aminoglycosides, the incidence of ototoxicity increases.
6. Nephrocalcinosis.
Contraindication:
Absolute: None except hypersensitivity.
Relative: Hypotension, hypovolemia, hypo-kalemia,
hyponatremia.
Alert: Furosemide may have to be stopped if
child develops diarrhoea or vomiting.
To be used with caution when using digoxin (avoid
hypokalemia) and aminoglycosides (higher risk of ototoxicity)
Monitoring parameters: Serum Na, K+,
Ca++ and blood sugar.
Torsemide
Is also a loop diuretic similar to furosemide, but is
more potent (10 mg of torsemide is equivalent to 40mg of furosemide), has
a higher bioavailability and a longer duration of action. In an open label
study on children, torsemide was considered better than furosemide for
control of heart failure(11). It is more expensive than furosemide.
Thiazides
These drugs act on distal convoluted tubule of the
nephron. Except for metolazone, thiazides are relatively milder diuretics
and are rarely used in the treatment of heart failure. Hydrochlorothiazide
is the most often used drug in this category. Primary indications for
thiazide diuretics are mild hypertension and edema. The dose of hydro-chlorthiazide
is 2 mg/kg/day in two divided doses. Like furosemide, it also causes
excretion of Na, K+ and chloride
along with water. Hydrochlorothiazide is available as 12.5 gm, 25 mg, 50
mg tab. The drug is quite inexpensive.
Metolazone is ten times more potent than
hydrochlorthiazide and is useful in resistant cases of hypertension and
heart failure. Intermittent doses of metalozone may help to overcome
diuretic resistance which may occur due to fluid overload, mesenteric
congestion (inadequate absorption) and low renal blood flow. The dose is
2.5-5 mg/day for adults and 0.2-0.4 mg/kg/day in children. Electrolytes
must be monitored closely.
Spironolactone
Sprinolactone is an aldosterone blocking agent, the
other such drug is eplerenone. These act on distal convoluted tubule of
the nephron, producing moderate diuresis with Na and chloride excretion
and sparing of K+. Spironolactone is
often used in combination with furosemide for heart failure.
Evidence: Sprinolactone has been shown to improve
survival in adult patients with heart failure(12). No such specific
benefit has been shown in children, but the drug is effective.
Two small observational studies using
spironolactone(13,14) in children, have shown benefit in controlling heart
failure.
Dosage and preparations: Neonates 1-3
mg/kg/d in 1-2 divided doses; Children 1.5-3.5 mg/kg/d in 1-4 divided
doses; Adult 25-200 mg in 1-2 divided doses. Available as 25 mg, 50 mg and
100 mg tablet.
Side Effects: Electrolyte imbalance: hyperkalemia,
especially when using with ACEi and in renal impairment; anorexia,
gastritis, gastric bleeding, diarrhea, gynaecomastia, irregular menses,
and amenorrhea. These side effects are dose and duration related and tend
to reverse after discontinuation of the drug. These are not seen with
eplerenone.
Contraindications: Significant renal failure,
hyperkalemia, peptic ulcer
Monitor: Serum K+,
renal functions, especially if renal impairment.
3. Vasodilators: Angiotensin Converting Enzyme
Inhibitors (ACEi)
ACEi decrease the adrenergic drive and block the heart
failure induced activation of renin angiotensin aldosterone axis (RAAS).
Increased levels of aldosterone and angiotension II have been associated
with poor outcome in heart failure. ACEi also increase bradykinin which
has natrinuretic properties. Currently ACEi therapy is recommended as the
first line treatment for heart failure, when it is not secondary to an
obstructive lesion.
Indications:
1. Heart failure due to ventricular dysfunction
2. Hypertension
3. Significant valvular regurgitation (even without
heart failure)
4. Heart failure secondary to large left to right
shunts: Role of ACEi is less convincing, but is often used.
Classification: ACEi are classified into 3 classes;
I Captopril is the active form of the drug and it is
metabolized in liver.
II Enalapril, ramipril: These are pro-drugs and are
metabolized to the active form.
III Lisinopril: Is excreted without being metabolized
by the kidney.
Evidence: Improvement in symptoms and survival has
been shown in adults with symptomatic heart failure on ACEi(15,16). Later,
ATLAS trial showed that high dose of lisinopril was more beneficial than a
low dose(17). Therefore, one must up titrate the dose to the maximum
tolerable permissible doses for maximum benefit.
There are no randomized trials in children, the trials
may be considered unethical at this stage. Several small observational
studies have proven the efficacy and safety of these drugs in
children(18-20). There is one study showing survival benefit with ACEi in
children with idiopathic dilated cardio-myopathy(21). ACEi have been found
to be useful in valvular regurgitation(22) and large left to right shunts,
if the systemic vascular resistance is elevated at the baseline(23).
Captopril. Most often used ACEi in pediatric
practice, especially in neonates and infants where enalapril may induce
renal dysfunction. The starting dose is 0.1 mg/kg/dose; it is gradually
increased to 0.5-1 mg/kg/dose three times a day (increase after every 4 to
5 doses). Maximum dose is 2 mg/kg/dose. BP and renal parameters should be
monitored when up titrating the dose.
Enalapril. Enalapril is useful for older
children. It is longer acting and given twice daily. The dose is 0.1-0.5
mg/kg/dose twice a day. The initial dose may be smaller. Monitoring is as
for captopril.
Ramipril and lisinopril are other ACEi, both are
commonly used for hypertension. The doses for heart failure in children
are not defined.
Side effects: Hypotension: It usually occurs in the
initial phase (4 or 5 doses) and recovers after reduction of the dose.
Cough is the most troublesome side effect. It is due to increased levels
of bradykinin. Non steroidal anti-inflammatory agents may be helpful. The
frequency of cough is lower in infants and children as compared to adults.
Monitoring: Blood pressure (BP), renal parameters,
serum K+ should be monitored,
initially and whenever the dose is increased. In a relatively stable
patient, ACEi therapy can be initiated in the outpatient department.
Alert: Avoid using ACEi with spirnolactone due to
possibility of inducing hyperkalemia.
Contraindications: Bilateral renal aretery stenosis,
to be used carefully in coarctation of aorta, renal failure with severe
decrease in GFR, hyperkalemia, preterm and sick neonates – avoid ACEi
especially enalapril, and pregnancy.
Hydralazine: It is a non ACEi peripheral
vasodilator, resulting in relaxation of arterial smooth muscles.
Hydralazine does not produce hyperkalemia, and is safe in patients with
renal impairment. It should be used in patients in whom ACEi or ARB are
not tolerated or are contraindicated. Dose is 0.75 mg/kg/day; may be
increased gradually up to maximum of 5 mg/kg/day in four divided doses. It
is available with difficulty.
4. Angiotension Receptor Blockers (ARB)
ARB are competitive antagonists for the angiotension II
receptors, they block the cell surface receptor for angiotension unlike
ACEi, which are converting enzyme inhibitors. ARB do not inhibit
bradykinin breakdown and hence cough is much rarer. Also ARB are not
nephrotoxic. However, a meta analysis of randomized trials in adults did
not show any advantage of ARB over ACEi(24).
Side effects are same as for ACEi except that cough
does not occur. Other drugs in this group besides the commonly used
losartan, are Candesartan and Valsartan. Studies in children are in
progress, primarily for treatment of hypertension. A combination of ACEi
and ARB is currently not recommended in pediatric patients.
Dose of Losartan: 0.75 to 1.4 mg/kg/day
5. Beta blockers
Heart failure results in activation of sympathetic
nervous system and increased levels of circulating catecholamines. Chronic
activation of sympathetic nervous system leads to worsening of heart
failure by inducing myocardial apoptosis and fibrosis. Circulating
catecholamines also induce peripheral vasoconstriction along with renal
retention of salt and water. Betablockers antagonize these deleterious
effects(25). In addition, betablockers also have antiarrhythmic effect.
Indications:
1. Mild, moderate or compensated heart failure,
secondary to ventricular dysfunction. Beta-blockers should not be
initiated in acute decompensated heart failure.
2. SVT and other tachyarrhythmias
3. Hypertension
Evidence: The benefits of betablocker therapy in
adult patients with heart failure have been shown in several studies(26).
In addition to metoprolol, carvedilol has been shown to decrease all cause
mortality and risk of clinical progression of heart failure(27,28).
Carvedilol is a non selective beta blocker which also has an anti-oxidant
property. Due to its alpha blocking effect, carvedilol exerts a
vasodilatory effect. It improves functional class and fractional
shortening in children with ventricular dysfunction(29). Side effects
include dizziness, hypotension and headache. The first multicentre,
randomized, double blind, placebo controlled trial for carvedilol in
children is recently published by Shaddy and colleagues(30). There was no
statistically significant difference between carve-dilol and placebo.
Authors postulated that this lack of effect may be due to unexpectedly low
rate of events for patients in worsened category and that the trial may
have been underpowered.
Pharmacodynamics: Dose reduction is required in
severe liver dysfunction, but not for renal dysfunction. Carvedilol
increases digoxin concentration so dose of digoxin may have to be
decreased by 25% when using carvedilol. Combination with calcium channel
blockers should be avoided.
Dosages:
Metoprolol: 0.2-0.4 mg/kg/day initially, gradually
increase to a maximum of 1 mg/kg/day in two divided doses.
Carvedilol: 0.1 mg/kg/day in two divided doses,
increase at 1-2 weekly interval to 1 mg/kg/day with a maximum of 2
mg/kg/day.
Metoprolol is available as 12.5 mg, 25 mg, 50 mg, and
100 mg tablet.
Carvedilol is available as 3.125 mg, 6.25 mg, 12.5 mg,
and 25 mg tablet.
Side effects: Bronchospasm, bradycardia, heart
block, hypotension, hyperglycemia, dizziness. Aggravation of heart failure
may occur in some cases, the diuretic dose may have to be increased.
Contraindications: Advanced heart block, sick sinus
syndrome, acute heart failure, bronchial asthma, cardiogenic shock.
Relative contraindications include chronic airway disease, bradycardia,
hypotension, hypothyroidism.
Anticoagulation for children with chronic heart failure
is discussed later.
B. Algorithm for Management of Heart Failure
Key concepts in management of heart failure in children
are listed in Box 1. In neonates and infants, active
fluid restriction is not recommended. Calorie supplementation, either by
increasing the density of milk or giving commercially available high
calorie formulas, is recommended. In older children, fluid and salt
restriction are generally required. Children should be asked to avoid
extra salt as is present in fries, chips, pizzas and other similar food
items. Drug therapy has to be individualized as per clinical setting A-D,
as described below:
Box 1 Guidelines for Management of Heart Failure
Do
• Treat the underlying cause of heart failure.
• Digoxin has a narrow safety window in children.
• Continuous infusion of furosemide may be better in acutely ill cases .
• A persistent tachycardia (>180) may indicate “tachycardiomyopathy” as the cause of heart failure.
• Rapid digitalization is not required for majority.
Do Not
• Combine angiotensin converting enzyme inhibitors (ACEi) with Angiotensin receptor blockers (ARB) (Class III).
• Avoid combining ACEi and spironolactone, if necessary, monitor potassium levels (Class II b)
• Do not give ACEi in heart failure secondary to pressure overload (Class III)
• Avoid using ACEi in acute decompensated heart failure (Class II b)
• Betablockers should not be initiated in acute decompensated stage of heart failure (Class III)
• Potassium supplements are not required in early infancy
|
Clinical setting A: Patients at
increased risk for heart failure, but no volume overload or ventricular
dysfunction as seen in exposure to cardiotoxic agents; family history of
heritable cardiomyopathy; univentricular hearts (pre and post Fontan);
congenitally corrected transposition.
Therapy consists of the following: (i) avoid
cardiotoxic drugs; (ii) periodic clinical assessment; (iii)
periodic echocardiographic evaluation for ventricular function; (iv)
maintenance of sinus rhythm. There is no role of ACE inhibitors/
Betablockers (Class III).
Clinical setting B: Patients with
abnormal cardiac morphology or function, but no symptoms of heart failure
as seen in mitral regurgitation (MR) or aortic regurgitation (AR) with
left ventricular enlargement; and univentricular heart with dysfunction.
Therapy and class of recommendation: See
Table III.
Table III
Drug Therapy and Class of Recommendation for Patients with Abnormal Cardiac
Morphology or Function, but no Symptoms of Heart Failure
| |
Preserved systolic
function |
Ventricular |
| |
Left to right shunts |
MR/ AR |
Dysfunction |
|
ACEi |
III |
IIa |
I |
|
Betablockers |
III |
III |
I (IIa if RV morphology) |
|
Digoxin |
III |
III |
III |
|
Diuretics |
III |
III |
III |
|
Anticoag |
III |
III |
IIb |
ACEi: angiotensin converting enzyme inhibitor; AR: aortic regurgitation; MR: mitral regurgitation; RV: right ventricle
|
Clinical setting C: Patients with past or
current symptoms of heart failure (commonest group)
Therapy and class of recommendation is detailed in
Table IV.
Clinical setting D: Treatment for
end-stage heart failure requiring continuous infusion of inotropic agents,
mechanical circulatory support, cardiac transplantation or hospice care.
Therapy: Intravenous infusion of dopamine,
dobutamine, milrinone, alone or in combination (details described later in
section on "Drugs in ICU setting"). Betablockers and ACEi should not be
used (Class III).
Table IV
Drug Therapy and Class of Recommendation for Patients with Past or Current
Symptoms of Heart Failure
| |
Preserved systolic
function |
Ventricular |
Pressure overload |
RV Dysfuction |
| |
Left to right shunts |
MR/ AR |
Dysfunction |
|
|
|
Diuretics |
I |
I |
I |
IIa |
I |
|
Digoxin |
IIa |
IIa |
I (for symptoms) |
III |
IIa |
|
ACEi |
IIa |
I |
I |
III |
I |
|
Betablockers |
III |
III |
IIa |
III |
IIb |
|
Anticoag |
III |
III |
IIa |
Urgent intervention to relieve obstruction |
|
ACEi: angiotensin converting enzyme inhibitor; AR: aortic regurgitation; MR: mitral regurgitation; RV: right ventricle
|
Hypertension
Systemic hypertension is an important, often
underdiagnosed, condition in children. In developed countries, the
estimated prevalence is 1%-2% during childhood. Small surveys in school
children suggest a prevalence of 2%-5% in India(31). It is recommended
that all children over 3 years of age should have their BP measured
whenever seen by a doctor. Hypertension in children can be essential
(primary) or secondary (often to renal or endocrine disorder). Readers are
encouraged to refer to the guidelines on "Evaluation and management of
hypertension" recently published in Indian Pediatrics(32).
Definition of Hypertension
Normal BP: systolic or diastolic BP < 90th
centile for gender, age and height.
Pre hypertension: systolic or diastolic BP between
90th & 95th centile.
Hypertension: systolic or diastolic BP exceeding
95th centile on 3 separate occasions. This is further subdivided into 2
stages
Stage I: Systolic or diastolic BP >95th centile and
up to 5 mm above the 99th percentile
Stage II: Systolic or diastolic BP values 5mm or
more above the 99th percentile
Anti Hypertensive agents
Before starting drug therapy, it is important to
differentiate essential or primary hypertension from secondary
hypertension. In most cases of secondary hypertension, treatment of the
underlying cause can cure the child of high BP and hence the need for long
term antihypertensive therapy. The doses of various antihypertensive
agents are given in Table V.
Table V
Oral Antihypertensive Medications
|
Drug |
Initial dose (maximum) |
|
Calcium channel blockers |
|
Amlodepine |
Children 6-17 years: 2.5-5mg once daily, od-bid |
|
Nifedipine (extended release) |
0.25-0.5mg/kg/d (3mg/kg/d up to 120mg/d), od-bid |
|
Isradipine |
0.15-0.2mg/kg/d (0.8mg/kg/d up to 20mg/d), tid-qid |
|
Angiotension converting enzyme inhibitors, angiotension receptor
blockers |
|
Captopril |
0.3-0.5mg/kg/d (6mg/kg/d), tid |
|
Enalapril |
0.08mg/kg/d up to 5 mg/d (0.6mg/kg/d up to 40mg/d) , od-bid |
|
Lisinopril |
0.07mg/kg/d up to 5mg/d (0.6mg/kg/d up to 40mg/d), od |
|
Ramipril |
6 mg/M2, od |
|
Irbesartan |
4-5mg/kg/d (6-12 years:150mg/d, >13 years: 300mg/d), od |
|
Losartan |
0.7mg/kg/d up to 50mg/d (1.4mg/kg/d up to 100mg/d), od |
|
Beta blockers |
|
Atenolol |
0.5-1mg/kg/d (2mg/kg/d up to 100mg/d), od-bid |
|
Metoprolol |
1-2mg/kg/d (6mg/kg/d up to 200mg/d), bid |
|
Propanolol |
1-2mg/kg/d (4mg/kg/d up to 640mg/d), bid-tid |
|
Labetalol |
1-3mg/kg/d (10-12mg/kg/d up to1200mg/d), bid |
|
Central alpha agonist |
|
Clonidine |
5-25 ug/kg/d (2.4mg/d), tid-qid |
|
Peripheral alpha antagonist |
|
Prazosin |
0.05-0.1mg/kg/d (0.5mg/kg/d), bid-tid |
|
Vasodilators |
|
Hydralazine |
0.75-1mg/kg/d (8mg/kg/d), qid |
|
Minoxidil |
0.1-1mg/kg/d (50mg/d),od-bid |
|
Diuretics |
|
Frusemide |
0.5-2mg/kg/d (6mg/kg/d),od-bid |
|
Spironolactone |
1mg/kg/d (3.3mg/kg/d up to 100mg/d), od-bid |
|
Metolazone |
0.2mg/kg/d (0.4mg/kg/d), od |
|
Hydrochlorothiazide |
1mg/kg/d (3mg/kg/d), od |
|
Amiloride |
0.4-0.6mg/kg/d (20mg/d), od |
od: once a day; bid: twice a day; tid: thrice a day; qid: four times a day
|
Algorithm for treatment of Hypertension
Life style modifications are very important; their
discussion is beyond the scope of this article, but they are required for
all stages of hypertension, with or without drug therapy. Drug therapy is
indicated in children with hypertension and in those with pre
hypertension, when associated with co-morbid conditions. The goal of
treatment is to reduce BP to below 95th
centile or below 90th centile, if target organ damage or a co-morbid
condition is present. Commonly used medications include ACEi, calcium
channel blockers (CCB), other vasodilators, beta blockers and thiazide
diuretics. Recommended algorithm for treatment of hypertension is:
· Initial
treatment with CCB or BB or ACEi
· If BP
continues to be >95th centile: Use combination therapy - ACEi + CCB
or ACEi + Thiazides or CCB + BB. (Watch for bradycardia when combining
BB and CCB)
· If BP
continues to be >95th centile: Add third agent - ACEi + CCB +
Diuretic/BB. Other agents: prazosin, clonidine, hydralazine. BB:
betablockers, CCB: calcium channel blockers
Choice of drugs according to the cause of hypertension
For essential hypertension in children, one can start
with a drug from the group of calcium channel blocker, ACEi or betablocker.
If hypertension is secondary to acute glomerulonephritis, loop diuretic
like furosemide along with a calcium channel blocker or ACEi should be
used. ACEi can be used for hypertension secondary to renal disease as long
as the GFR is >30 mL/hour. If it is <30 mL/hour, calcium channel blocker
and/or betablocker should be used instead of ACEi. It is important to
monitor serum potassium and creatinine levels in such cases. For
renovascular hypertension, ACEi should not be used if renal artery anatomy
is not clear or if there is bilateral renal artery stenosis. A combination
of a calcium channel blocker and a diuretic should be used. One can use a
betablocker instead of a calcium channel blocker if ventricular function
is normal or mildly deranged. Same is true for hypertension secondary to
coarctation of aorta.
Anti-thrombotic, Antiplatelet And Thrombolytic Therapy
Recommendations for anti-thrombotic therapy in children
have been extrapolated from experience in adult patients, perhaps due to
relative infrequency of thromboembolic events in children. Anti-thrombotic
agents are required in critically ill-neonates with umbilical catheters,
children with cancer (requiring long term indwelling lines), children with
pro-coagulation abnormalities, post cardiac surgery etc.
1. Heparin
Unfractionated heparin is the most commonly used
anticoagulant. It acts by forming a complex with antithrombin III which
inhibits coagulation factors IX, X, XI, XII, plasmin and kallikrin.
Heparin also binds to a glycoprotan, cofactor II that inactivates thrombin
independently of antithrombin III. Neonates and infants have reduced level
of antithrombin III and have faster clearance of heparin, both these facts
result in increased dose requirements in pediatric group. Resistance to
heparin can be overcome by increasing either the dose of heparin or the
antithrombin concentration(33). Heparin dose is titrated to achieve an
activated partial thrombo-plastin time (aPTT) of 60-85 seconds which
generally correlates with anti Xa level of 0.35-0.7 unit per mL.
Indications: Heparin is used as first line
anticoagulant until oral agents such as warfarin are initiated. Heparin
bolus is given in the catheteri-zation lab to prevent risk of arterial
thrombosis. Heparinised saline is used to flush catheters in the
catheterization lab.
Dosage: An IV bolus dose of 75-100 units/kg of
heparin results in a therapeutic aPTT in 90% of children.
Maintenance dose (as IV infusion)
< 2 months of age 28 units/kg/hour
2 mo-1yr 25 units/kg/hour
> 1 yr 20 units/kg/hour
Older children 18 units/kg/hour (same dose
as for adults)
Dosage in catheterization lab: 50-100 units/kg bolus IV
or through arterial sheath.
Monitoring: Heparin dosing monograms have been
validated in children(34). Many physicians use anti Xa levels for infants
or in critically ill children as aPTT may not be very predictive. In
relatively stable infants and in older children, aPTT is used for
monitoring as it is more easily performed and is widely available. If aPTT
is <60 seconds, dose of heparin should be increased by 10% every 4-6 hours
till aPTT is over 60 seconds. If aPTT exceeds 85 seconds, heparin dose
should be decreased and if aPTT is >95 sec, the heparin infusion should be
stopped.
Side effects: Bleeding occurs in 1.5%-24%, the true
frequency may be somewhere in between these figures. Reports of heparin
induced thrombo-cytopenia have been described in up to 2.3% for children
in intensive care. Osteoporosis is rarely seen, it is related to long
duration of use. Protamin sulfate is used to counter effect of heparin
immediately, in case bleeding occurs. The dose is 1 mg for every 100 units
of heparin, if heparin infusion has been received in past 30 minutes.
Reduced doses of protamin sulfate are required if the last heparin
infusion given was over 30 minutes ago.
2. Low Molecular Weight Heparin
Low molecular weight heparin (LMWH) is
increasingly used in children, primarily due to less stringent monitoring
requirement. The mechanism of action is similar to that of heparin.
Studies from Hospital for Sick Children in Toronto confirm the advantages
of LMWH over standard heparin(35,36). These advantages are relative ease
of subcutaneous administration, minimal need for monitoring, mini-mal
interference by other concurrent medications, decrease effect on bones
with long term use and decreased incidence of thrombocytopenia. Several
studies have confirmed the efficacy of LMWH in children and a need for
higher dose has been confirmed in these studies, when compared to
adults(37-39). Therapeutic dose is extrapolated from adults and is based
on anti factor Xa levels. The recommended level of Xa is 0.50-1.0 unit/mL
when sample is taken after 4-6 hours of a subcutaneous injection.
Dosage: Majority of data in children is with
enoxaprin. Dose is 1.5mg/kg 12 hourly for <2 month (<5kg) and 1 mg 12
hourly for older infants and children. For preterm babies a higher dose,
up to 1.5 – 2 mg/kg 12 hourly, may be required.
Adverse reactions include major bleeding (4%).
3. Vitamin K Antagonists
These drugs reduce the concentration of vitamin K
dependent factors, II, VII, IX and X. In newborns, concentration of these
factors is physiologically reduced, resulting in a prolonged prothrombin
time, the international normalized ratio (INR) being 2.0-3.0. So, the role
of vitamin K antagonists is limited in newborn period. The breast milk has
a low concentration of vitamin K, making breast fed infants very sensitive
to these drugs. On the other hand, formula milk has high vitamin K levels
and therefore, babies on formula milk are relatively resistant to vitamin
K antagonists.
Warfarin and acenocoumerol are available drugs in this
category. Only oral preparations are available in the market. Warfarin is
the most common oral anticoagulant used; it is indicated for prophylaxis
and treatment of thromboembolic disorders. The efficacy of warfarin is
judged by measuring INR.
Dosage: Initial loading dose is 0.2 mg/kg. Infants
<1 yr usually need higher maintenance dose compared to older children. The
dosage schedule is as per INR value given in the Table VI.
Average dose of warfarin in infants and young children is 0.33 mg/kg/day
to achieve an INR of 2.0-3.0. For teenagers, the dose is 0.09 mg/kg/day
and for adults, 0.04-0.08 mg/kg/day.
Table VI
Dosing of Warfarin
|
Loading dose (Day 1) |
|
0.2mg/kg (maximum 10mg); 0.1 mg/kg in
presence of hepatic dysfunction |
|
Days 2-4 |
|
INR 1.1-1.3, repeat loading dose |
|
INR 1.4-1.9, give 50% of initial loading
dose |
|
INR 2.0-3.0, give 50% of initial loading
dose |
|
INR 3.1-3.5, give 25% of initial loading
dose |
|
INR > 3.5, hold until < 3.5, restart at
50% of previous dose |
|
Maintenance dose (day 5 and beyond) |
|
INR 1.1-1.4, increase dose by 20% of
previous dose |
|
INR 1.5-1.9, increase dose by 10% of
previous dose |
|
INR 2.0-3.0, no change |
|
INR 3.1-3.5, decrease dose by 10% of
previous dose |
|
INR >3.5, hold until <3.5, restart at 20%
of previous dose |
INR:
international normalized ratio
|
Monitoring: Frequent dose adjustments warrant close
supervision of INR. Vitamin K antagonists have extensive cross-reactivity
with several commonly used drugs and dietary agents. Certain
"point-of-care" monitors are commercially avai-lable, which are considered
reliable and acceptable for checking INR in home setting. These are
somewhat similar to "glucometers".
Side effects: Bleeding is the main complication;
the risk of major bleeding is 0.5% per patient year. Risk increases
significantly when INR is >8 units. Other side effects are development of
osteoporosis on prolonged use. The complication of bleeding can be treated
with vitamin K administration (30 mcg/kg). In serious cases, fresh frozen
plasma should be used.
Contraindications: These include severe
renal or hepatic impairment, cerebral or dissecting aortic aneurysms,
active ulceration, severe hypertension, infective endocarditis,
pericardial effusion, preg-nancy and hypersensitivity to warfarin.
Interactions: A major concern in using
vitamin K antagonists is their interaction with several drugs and dietary
substances, requiring dose adjustments. The dose of warfarin needs to be
increased when using anticonvulsants like phenobarbital and carbamaze-pine.
Other drugs interacting with warfarin are aspirin, steroids, nonsteroidal
anti inflammatory agents, alcohol, fluconazole, metronidazole,
amoxi-cillin, rifampicin, chloramphenicol, sulfametho-xazole-trimethoprim
combination etc. As mentioned earlier, breast fed infants are more
sensitive to warfarin as compared to formula fed infants.
Patients should be told not to make frequent changes in
their diet. If a new drug is needed e.g. antibiotics, INR should be
monitored. INR level is affected by cumulative dose of warfarin taken over
the last 5-7 days, so testing INR just after a day of change in dose is
not very useful.
4. Antiplatelet Agents
Aspirin
The effect of aspirin is mediated through inhibition of
prostaglandin synthetase, which results in prevention of formation of the
platelet aggregating substance thromboxane A2. This antiplatelet effect is
generally seen at doses of 3-5 mg/kg/day. Aspirin resistance, as seen in
adults is prevalent in children also, 26% in one study(40). The main
indications in children are, following palliative Blalock Taussig (BT)
shunts and in patients with Kawasaki syndrome. In a nonrandomized
observational study, aspirin was found to lower the risk of death and BT
shunt occlusion(41). Low dose aspirin has also been used following Glenn
and Fontan procedures. Aspirin clearance is slower in neonates. Aspirin
should be administered with milk or food as it may cause gastric
irritation. A controlled release preparation must not be crushed or chewed
as the bioavailability will change. Generally no specific monitoring is
required when using low dose aspirin, however hemoglobin may be checked
every 6 months to detect anemia which may occur due to blood loss from
gut.
Side effects: Aspirin is relatively safe in anti
platelet doses. Rarely it may result in bleeding, especially in those with
underlying coagulation disorder. Symptoms of peptic ulcer may be
precipitated. Reye syndrome is dose dependent, hence not seen with low
dose aspirin. In the rare event of significant bleeding, platelets should
be transfused.
Clopidogrel
It is one of the thienopyridines and selectively
inhibits ADP-induced platelet aggregation via the inhibition of P2Y12
receptor. Its effect is additive to anti platelet effect of aspirin.
Data in children on the use of clopidogrel is emerging.
The initial report was on 15 children(42). More recently there is a
prospective, multicenter, randomized, placebo controlled trial (PICOLO)
that was conducted to evaluate the pharmacodynamics of clopidogrel in 116
children with risk for arterial thrombosis(43). The drug was well
tolerated and a dose of 0.2 mg/kg/day was able to achieve platelet
inhibition level similar to that in adults taking the standard dose of
75mg/day. 80% of children were also taking aspirin, no serious bleeding
occurred.
The indications for clopidogrel are same as for
aspirin. It may be considered in cases that are intolerant to aspirin.
Clopidogrel can be combined with aspirin in cases where stronger
antiplatelet effect is required.
Side effects: Frequency of side effects is low.
Main side effects are gastrointestinal. Rarely rash, neutropenia and
bleeding has been described in adults.
Ticlopidin is another thienopyridine, given in
doses of 10 mg/kg/day in two divided doses, but there is no data to
support its use in children.
Intravenous antiplatelet agents
These include glycoprotein IIb-IIIa antagonists such as
intravenous abciximab, eptifibatide and tirofiban. One study on use of
abciximab for patients with Kawasaki disease demonstrated greater
reduction in coronary aneurysm diameter at early follow up compared to
patients who received standard therapy alone(44). Abciximab therapy may be
considered in patients of Kawasaki disease who develop large coronary
aneurysms in acute or sub acute phase.
5. Thrombolytic Agents
Thrombolytic agents act by converting endogenous
plasminogen to plasmin. The various agents in this group are
streptokinase, urokinase and tissue plasminogan activater (tPA). Levels of
plasminogen are much lower at birth (50% of adult values). Therefore the
thrombolytic effect of these drugs is decreased in neonates. Streptokinase
is the cheapest of all three agents but may produce allergy in some cases.
In Western countries, tPA is the agent of choice. A review of 182 neonates
and infants given thrombolytic agents failed to show any significant
difference between the three agents(45). Fresh frozen plasma
supplementation may be used to increase efficacy of tPA.
Indications include femoral artery occlusion
(following cardiac catheterization), aortic throm-bosis, intracardiac
thrombi, pulmonary embolism, thrombosed prosthetic valves and thrombosed
BT shunts.
Dosage: The optimal dose for pediatric patients is
not known. Table VII outlines the usual dosage schedule and
monitoring parameters. These drugs are generally administered
intravenously; however local therapy may be better for catheter induced
thrombosis, if the catheter is already in situ. It is important to start
heparin therapy immediately after completion of thrombolytic therapy, a
loading dose for heparin is not required.
Table VII
Dosage for Thrombolytic Agents
| |
Loading |
Maintenance |
Monitoring |
| Urokinase
|
4,400u/kg |
4,400 u/kg/h for 6-12h |
Fibrinogen, TCT, PT, aPTT |
|
Streptokinase |
2,000 u/kg |
2,000 u/kg/h for 6-12h |
Fibrinogen,
TCT, PT, aPTT |
| tPA |
None |
0.1-0.6mg/kg/h for 6h |
Fibrinogen, TCT, PT , aPTT |
|
aPTT: activated partial thromboplastin tim;, INR:
international normalized ratio; PT: prothrombin time; TCT: thrombin
clothing time; tPA: tissue plasminogen activator
|
Side effects: The major adverse effect is bleeding,
seen in 20%-68% of cases. A higher dose and a long duration of therapy,
predispose to bleeding. Intracranial bleed has been reported in 4% of
pre-term babies as compared to 1% in term babies(46). Major bleeding may
be treated with cryoprecipitate and other blood products.
Contraindications include stroke, transient
ischemic attacks and severe hypertension.
Guidelines for Use of Anti-thrombotic Agents
1. Blalock – Taussig Shunts
Modified BT shunt involves interposition of a Gortex
tube between the subclavian artery and ipsilateral branch of pulmonary
artery. The risk of thrombotic occlusion of graft varies from 1%-17%
depending on various factors such as size of the graft, size of the
pulmonary artery, age of the patient, hematocrit, etc.
Recommendations: Heparin should be used during and
immediately following a BT shunt. This should be followed by aspirin in a
dose of 3-5 mg/kg/day (Class I). Clopidogrel may be used in place
of aspirin in those unable to tolerate aspirin (Class IIa). A
combination of aspirin and clopidogrel may be used if one episode of shunt
thrombosis has occurred on aspirin alone (Class IIa).
2. Mechanical Valves
Thrombosis of a prosthetic valve can be catastrophic
and must be prevented. Warfarin is a very effective oral anticoagulant to
prevent prosthetic valve throm-bosis in adults; data in children is less
robust.
Recommendations: Warfarin (or other vitamin K
antagonists) to be used for all children with mechanical valves (Class
I). The recommended INR is 2.5-3.5 for prosthetic mitral valve and
2.0-3.0 for prosthetic aortic valve. The dose of oral anti-coagulant
should be titrated accordingly.
For those who have had a valve thrombosis while on
adequate oral anticoagulation, addition of aspirin, in a dose of 3-5
mg/kg/day should be considered. (Class IIa).
For a bioprosthetic valve, oral anticoagulation to
maintain INR between 2.0 and 3.0 is recommended for initial three months
after surgery, no anticoagulation is required thereafter. Low dose aspirin
may be used (Class IIb).
3. Kawasaki Disease
Coronary artery aneurysms develop in 15%-25% of
patients with Kawasaki disease. Treatment with high dose intravenous gamma
globulins has been shown to reduce the risk of coronary aneurysm.
Antiplatelet agents are used to prevent coronary thrombosis in acute phase
and myocardial infarction in chronic phase.
Recommendations: Aspirin, in anti inflammatory dose
of 80 -100 mg/kg/day is used for initial phase, sometimes up to 14 days (Class
I). Later, the dose is reduced to 3-5 mg/kg/day to exert antiplatelet
effect. It is given for 6-8 weeks if no coronary abnormalities are present
(Class I). If coronary aneurysms are present, aspirin in low doses
is continued till the aneurysms persist, which may be life long (Class
I). For big coronary aneurysms, (over 6-8 mm in diameter), clopidogrel
may be added to aspirin therapy (Class IIb). For giant aneurysms
(>8mm in diameter), addition of oral anticoagulation is recommended to
aspirin therapy (Class I). The target INR should be maintained
between 2.0 and 3.0. For coronary artery thrombosis, glycoprotein IIb IIIa
inhibitors like abciximab may be used (Class IIa). Abciximab may
also be indicated for giant aneurysms (Class IIb).
4. Intracardiac Thrombi in Neonates with Normal
Ventricular Function
Neonates are particularly vulnerable to intracardiac
thrombi due to imbalances in their fibrinolytic systems and low levels of
natural anticoagulants in their body.
Recommendations: Direct infusion of the
thrombolytic agent as close to thrombus as possible is preferred (Class
I). If given IV, higher doses are required, which increase the risk of
cerebral hemorrhage, more so in preterm babies. The dose for urokinase is
1000 to 3000 units/kg/hour and for tPA 0.01-0.05 mg/kg/hour(47).
Fibrinogen levels should remain above 100 mg/dl during treatment. The
thrombolytic treatment should be followed by heparin infusion.
5. Dilated Cardiomyopathy/myocarditis
Dilated cardiomyopathy or myocarditis with heart
failure predisposes to stroke and pulmonary embolism.
Recommendations: Those with gross heart failure
should receive oral anticoagulants (Class I). Oral anticoagulants
are also preferred for other children with cardiomyopathy who have
significant ventricular dysfunction (Class IIa). The target INR is
kept between 2.0 and 3.0. If intracavitary thrombus is present,
anticoagulant therapy is again warranted (Class I).
6. Idiopathic Pulmonary Hypertension
Anticoagulants are often used for prophylaxis in this
group of patients, based on the data generated in adults.
Recommendations: Oral anticoagulation with vitamin
K antagonists to maintain INR between 2.0 and 3.0 (Class IIa).
Antiplatelet agents have no role.
7. Arterial Cardiac Catheterization (Diagnostic and
Interventional)
Young children are at increased risk of femoral artery
thrombosis following arterial access for cardiac catheterization. Femoral
artery thrombosis should be suspected if the pulse in the corresponding
limb remains absent after 2-4 hours of the procedure and it should be
treated with anticoagulants.
Prophylaxis for femoral artery thrombosis
Use of heparin during cardiac catheterization is shown
to reduce incidence of femoral artery thrombosis in children by 40% to
80%. In a study, 50units/kg bolus was found to be as effective as
100units/kg, given immediately after arterial puncture(48). However most
recommend the higher bolus dose of 100units/kg in infants and young
children (Class I). If procedure is prolonged, additional doses of
heparin or a heparin infusion is used. Activated clothing time should be
monitored if the procedure is prolonged; it is maintained between 200-250
seconds.
Treatment of femoral artery thrombosis
If pulse in the index limb does not appear after 2-4
hours of cardiac catheterization, heparin should be given in a dose of 20
units/kg/hour (Class I). If pulse still remains absent after 36-48
hours, thrombolytic therapy is recommended (Class IIa). For
significantly ischemic limb (threatening to extend or limb death),
thrombolytic therapy may be initiated early (Class I). IV
streptokinase is used, the bolus dose is 1000-4000 units/kg given over
20-30 minutes. This should be followed by infusion at 1000 units/kg/hour.
If thrombolytic therapy is contraindicated, surgery should be done. It is
believed that in about 70% of cases, heparin resolves the thrombus.
8. Peripheral and Umbilical Arterial Catheter in
Neonates and Children
For prophylaxis against catheter thrombosis, heparin
infusion in concentration of 5units/mL should be continued through the
catheter at a rate of 1mL/hour. In case thrombosis has occurred in the
arterial catheter, the catheter should be removed and heparin infusion
given intravenously. In cases with significant limb ischemia, thrombolytic
agents may have to be used.
Pharmacotherapy For Arrhythmias
A. Supraventricular Tachycardia
SVT is the most frequent form of symptomatic
tachyarrhythmia in children. The heart rate is usually more than 180-200
bpm. The commonest type is due to an accessory connection between the
atrium and ventricle; it is called atrioventricular re-enterant
tachycardia (AVRT). Atrioventricular nodal reentrant tachycardia (AVNRT)
is less common in children. The least common type is ectopic atrial
tachycardia (EAT). SVT is poorly tolerated in neonates and infants,
leading to heart failure. Palpitation is the main symptom in older
children and adolescents. Most SVT patients have a structurally normal
heart. In rare instances, a persistent, chronic SVT may cause ventricular
dysfunction and a dilated cardiomyopathy like picture. Drugs for
pharmacological treatment of SVT include adenosine (for acute episode
only), calcium channel blockers like verapamil and diltiazem, digoxin,
betablockers, amiodarone, sotalol, flecainide and propafenone.
Antiarrhythmic drugs are classified into four categories based on their
mechanism of action (Table VIII). Digoxin and betablockers
(already discussed) are the most commonly used drugs for SVT. Other drugs
like amiodarone, sotalol and flecainide will be discussed in this section.
Table VIII
Vaughan Williams Classification of Antiarrhythmic Drugs
| Class |
Mechanism of action |
Drugs |
| I |
Sodium channel blockade |
|
| |
Ia Prolong repolarization |
Quinidine, procainamide, disopyramide |
| |
Ib Shorten repolarization |
Lidocaine, mexiletine, tocainide, Phenytoin |
| |
Ic Little effect on
repolarization |
Encainide, flecainide, propafenone |
| II |
Beta adrenergic blockade |
Propanolol, esmolol, acebutolol, I-sotalol |
| III |
Prolong repolarization (potassium channel blockade)
|
Amiodarone, bretylium, d,l-sotalol, ibutilide |
| IV |
Calcium channel blockade |
Verapamil, diltiazem, bepridil |
1. Amiodarone
Amiodarone has been used as an antiarrhythmic agent
since 1970s and is useful for both SVT and VT. It is primarily a class III
anti-arrhythmic drug, but has other class effects also. Oral amiodarone
usually takes days to exert its antiarrhythmic effect, but IV amiodarone
has immediate onset of action and can be used in acute settings. The
predominant effect after IV administration is due to its betablocking and
calcium channel blocking actions. Class III antiarryhthmic effect takes
much longer.
Indications: Amiodarone is indicated for difficult
to control SVT, which may be due to AVRT, AVNRT, atrial flutter or AF.
Amiodarone is the drug for choice for junctional ectopic tachycardia (JET)
which may occur in postoperative setting in the intensive care unit. This
drug is also useful for various types of VT.
Evidence: Several studies have been reported
on oral and intravenous amiodarone use in children(49-51). It has also
been successfully used in combination with flecainide(52) and
propranolol(53) for refractory tachyarrhythmias in infants and children.
Dosage: IV - Loading dose is 5mg /kg given
over 20-30min, it is followed by 5-15 mcg/kg/min infusion. In rare cases,
a higher loading dose, up to a maximum of 15 mg/kg, can be given. Oral -
Loading dose is 5 mg/kg given 2-3 times a day (maximum 200 mg/dose) for 5
days followed by 5 mg/kg/day as a single dose.
Amiadarone has a long duration of action and may exert
effect for weeks or months after discontinuation. Since it is metabolized
in liver, its dose must be adjusted in hepatic dysfunction.
Side effects: Very toxic, especially on chronic
usage. Side effects are seen in up to 75% of cases. IV amiodarone may
result in hypotension, nausea, sweating, and hot flushes.
Side effects of oral amiodarone are:
• Cardiac:
bradycardia, prolongation of QT interval, myocardial depression
• Thyroid:
hypothyroidism or hyperthyroidism
• Pulmonary:
pulmonary alveolitis, pneumonitis and
fibrosis
• Nervous system:
peripheral neuropathy,
vertigo, headache, insomnia
• Skin:
rashes, photosensitivity
• Eyes:
corneal deposits, optic neuropathy
• Liver:
jaundice may occur due to hepatic toxicity.
Interaction with other drugs: Amiodarone decreases
the clearance of digoxin, flecainide, procainamide and warfarin. There is
increased risk of ventricular arrhythmias when given with erythromycin.
Contraindications: Hepatic dysfunction, restrictive
lung disease, long QT interval.
Monitoring: BP monitoring when using IV amiodarone,
especially in patients with ventricular dysfunction. Periodic ECGs must be
done for QT interval. Testing for thyroid functions (before starting
amiadarone, after loading dose and 6 monthly), liver functions (before,
after loading and 6 monthly), pulmonary functions, chest X-ray
(before and 3-6 monthly later) and slit lamp examination of the eyes
should be carried out periodically.
Amiodarone, though a very effective antiarrhythmic
agent, must be avoided as it results in serious side effects, especially
for chronic use. Unfortunately due to difficulties in procuring other,
safer antiarrhythmics, amiodarone is being widely used in India. It should
be reserved for refractory arrhythmias not responding to simpler
medications.
Amiodarone is available as 100mg and 200mg tablet and
50mg/mL injection.
2. Sotalol
Sotalol is a mixture of D-and L-isoform. It is
primarily a class III antiarrhythmic agent, but has some class II (beta
blockade) effect also, due to L-isoform. It prolongs action potential
duration and results in lengthening of QTc interval. It exerts a negative
inotropic and chronotropic effect and reduces AV nodal conduction.
Indications: Sotalol is indicated for refractory
atrial tachyarrhythmias. It is often used for arrhythmias in postoperative
patients. For ventricular arrythmias, sotalol has been shown to be
superior to class I agents. It is preferable to amiodarone due to less
serious side effects. Sotalol has also been used for fetal arrhythmias
successfully.
Evidence: Experience with sotalol in
children is limited, most studies are observational or retrospective,
reporting a success in 90% for SVT(54,55). In refractory cases, it can be
combined with flecainide(56).
Dosage: Sotalol is given orally in a dose of 2-4
mg/kg/day in two divided doses. Doses up to 8 mg/kg/day have been used.
Body surface area is reported to be a better predictor for sotalol dosing;
the recommended dose is 30-70 mg/m2/day(57).
Side effects: Sotalol is a relatively safe drug
apart from its proarrhythmic effect seen in 3%-5%, due to prolongation of
QTc interval. Bronchospasm may occur in predisposed patients, due to its
beta blocking effect.
Contraindications: Long QTc interval (>450 msec) is
an absolute contraindication. Dosage adjustment is required in renal
failure. Hypokalemia and hypomagnesemia are other relative
contraindi-cations. It should be used with caution in patients with
significant ventricular dysfunction and heart failure.
Monitoring: Sotalol should always be initiated in
the hospital setting. A baseline QTc interval should be measured on ECG.
QTc should be monitored for at least 3 days after initiation of therapy
and again whenever the dose is increased. If QTc interval increases to >
500 msec, the dose should be reduced or drug stopped.
Sotalol is available as 40 mg tablet.
3. Flecainide
Flecainide is a class Ic antiarrhythmic and acts by
inhibition of the fast Na channel, prolongation of the action potential
duration and inhibition of the rapid repolarization current. Its greatest
effect is on His-purkinje system and ventricular myocardium producing
prolongation of QRS duration. Flecainide is a negative inotropic and may
induce ventricular arrhythmia in patients with significant myocardial
dysfunction.
Indications: Flecainide is indicated for chronic
prophylaxis of SVT in cases refractory to conventional drugs like digoxin,
betablockers and calcium channel blockers. Intravenous preparation has
been used for termination of acute episode of SVT. It is particularly
useful for treatment of automatic atrial tachycardia and JET. It is also
used for atrial flutter, postoperative intraatrial reentrant tachy-cardia
and VT. Flecainide should be initiated by a cardiologist or
electrophysiologist, who is familiar with its usage and side effects.
Evidence: Flecainide has been extensively used for
chronic prophylaxis of SVT in children with no underlying heart disease or
ischemia. It is effective in over 90% of cases(58,59). Flecainide has also
been used successfully in combination with amio-darone(52) and
sotalol(56). Flecanide is also useful for fetal SVT.
Dosage: IV dose for aborting an acute episode of
SVT is 2 mg/kg over 30 minutes. For maintenance, 100-250 mcg/kg/hour
infusion is given. The oral dose ranges from 2-8 mg/kg/day in 2-3 divided
doses, usual dose is 2-4 mg/kg/day. Dose according to the body surface
area is 50mg/m2/day for children
under two years and 80mg/m2/day for over two years.
The drug is metabolized in liver and 30-40% is excreted
unchanged in urine. Half life is about 20 hours, but it is shorter in
neonates. Drug takes about 3-5 days to reach a steady state level after
oral administration. Milk inhibits absorption of flecai-nide. It is
advisable to avoid milk consumption one hour before and after flecainide
administration.
Side effects: These include bodyache, asthenia,
tremors, headache, fatigue, agitation and gastrointestinal upset. The most
dreaded side effect is proarrhythmia, seen in 7-8% of cases. Proarrhythmia
is more likely if there is myocardial ischemia or ventricular dysfunction.
Drug interaction: Digoxin increases level of
flecainide. When using with amiodarone, flecainide dose should be reduced
by about 50%.
Contraindications: These include significant
valvular heart disease, ventricular dysfunction, heart failure, hypoxia,
recent myocardial infarction/ischemia, heart blocks, sinus node
dysfunction and bundle branch block.
Monitoring: Flecainide should be started in a
hospital setting. The QRS duration needs to be monitored meticulously. A
10% increase is expected. An increase of >25% in QRS width should be an
indication to reduce the dose or stop the drug. QRS width is best judged
when ECG is taken at a faster paper speed (50mm/sec or 100mm/sec). Plasma
levels of flecainide should be monitored ideally, especially in hepatic or
renal impairment, but this facility is not yet available in India.
Flecainide is available with difficulty in India.
The tablet strength is 50 mg and 100mg.
B. Algorithm for Treatment of Supraventricular
Tachycardia in Children
Natural history of SVT is different in infants and
children as compared to adults and it influences the long term
pharmacotherapy for these patients. 40%-70% of infants do not need drug
therapy for SVT beyond infancy, although many of these still have an
inducible SVT on electrophysiologic study. About one third of SVT patients
lose accessory pathway but 30% of these may develop recurrence of SVT at
8-10 years of age. Therefore, infants with SVT due to an accessory pathway
(AVRT), such as Wolff Parkinson White (WPW) syndrome, can wait for
spontaneous resolution of the pathway.
The treatment of SVT is described under two
subheadings, abolition of the acute attack and chronic prophylaxis.
Acute Management of SVT
(i) Vagal maneuvers: In a
hemodynamically stable child, vagal maneuvers should be tried before
pharmacologic therapy. Such maneuvers include ice bag application on the
face of infants, pressure on infant’s abdomen and gagging. Ice bag
application is most commonly used and it has an efficacy of up to 90%. A
plastic bag with ice cubes and water should be applied on child’s face
for 10 seconds at a time. Vagal stimulation by applying ocular pressure
is contraindicated in infants and young children (Class III).
(ii) Adenosine: Intravenous adenosine
is considered the drug of choice for acute termination of SVT. It is
effective in 95% of cases of re-entrant tachycardia, although success
rate in neonates may be lower. Adenosine has a very short duration of
action and therefore recurrence of SVT is common. The dose is 0.1-0.2
mg/kg, given as a rapid IV bolus. A 5 to10ml of saline should be pushed
immediately after giving adenosine bolus. Adenosine breaks SVT by
producing block at the AV node level.
(iii) Intravenous Verapamil/ Diltiazem:
These drugs have >90% efficacy for terminating reentrant SVT i.e. AVRT
and AVNRT. Verapamil or diltiazem should not be used in small children
(< 4 years) and in the presence of heart failure or pre-excitation on
the ECG. Verapamil or.diltiazem is rarely used now, since adenosine is
available. These drugs still have place in older children who show
recurrence of SVT after adenosine administration.
(iv) Esmolol: Intravenous esmolol (a
betablocker) has been used to terminate SVT in some cases with moderate
success.
(v) Intravenous Amiadarone: IV
amiodarone reverts most of reentrant tachycardia which use AV node for
their sustenance i.e. AVRT and AVNRT. This drug should be reserved for
resistant and recurrent SVT.
(vi) Ohers: Intravenous/oral Flecainide
has also been used to terminate SVT.
Chronic Prophylaxis for SVT
Chronic prophylaxis with drugs may be required for
short periods, as spontaneous resolution occurs in a significant number of
cases who present in infancy. Choice of the drug depends on the
tachycardia mechanisms, age of the child, associated structural heart
disease, ventricular function and familiarity of the physician with the
drug. The algorithm based on above factors is given in Fig.1
and 2. As can be seen in figures, initial treatment must start
with one of the safe drugs like a betablocker or digoxin.
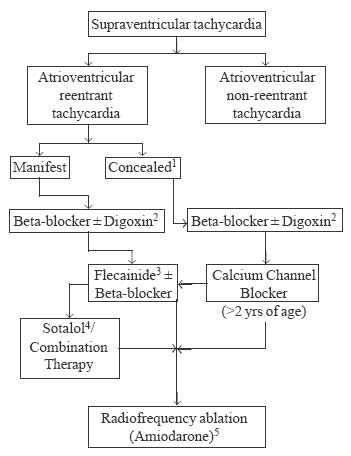 |
|
Fig.1 Management algorithm for children
with regular narrow QRS, reentrant SVT.1
Rarely fatal, hence does not warrant drugs like amiodarone / sotalol
on long term basis (>6 months);2 Use of Digoxin in manifest pathways
remains debatable; probably safe for infants (<2yrs);3
Interchangeable with propafenone;4 Always initiate in hospital; 3-5%
risk of Torsades de Pointes even in normal hearts;5 Amiodarone may
be used in special situations like uncontrolled recurrent episodes
in infants, severe LV dysfunction (tachycardio-myopathy), or when it
is difficult to monitor drugs like flecanide/sotalol etc. |
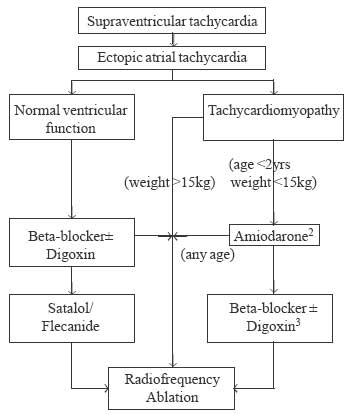 |
|
Fig. 2 Treatment algorithm for management
of Ectopic atrial tachycardia (EAT).1
In small children with severe LV dysfunction (EF <20%) short term
amiodarone (<1 yr) can be used to control the tachycardia. RF
ablation can be performed for these children if the tachycardia
continues to occur at a later age. Older children (>15 kg) can have
a RF ablation at the first instance or treated with amiodarone as in
small children.2 Amiodarone is highly successful in managing EAT and
thus improving LV dysfunction. If the tachycardia does not get
controlled with amiodarone, RF ablation can be performed even in
small infants.3 Once LV function normalizes, a trial of â-blocker ±
Digoxin, or Flecainide/Sotalol can be given till either the
tachycardia spontaneously disappears (upto 30%), or RF ablation can
be performed safely. |
AVRT is the commonest cause of SVT in infants. The
accessory pathway in such cases may be concealed or manifest. If the
accessory pathway is concealed, it is not capable of antegrade conduction.
ECG in such cases shows normal PR interval and no delta waves. Manifest
pathway is seen in 70% of cases, i.e. the pathway is capable of antegrade
conduction and the ECG shows short PR interval and delta waves.
Some of the key concepts regarding antiarrhythmic drugs
are given in Box 2.
|
BOX 2 -
Key Concepts in Management of Arrhythmias |
 |
Drugs In Intensive Care Sett | | |

