|
|
|
Indian Pediatr 2015;52:
763-767 |
 |
Pre-exchange Albumin
Administration in Neonates with Hyperbilirubinemia:
A Randomized Controlled Trial
|
|
Nabaneeta Dash, Praveen Kumar, Venkataseshan Sundaram
and Savita Verma Attri
From Department of Pediatrics, Advanced Pediatrics
Centre, PGIMER, Chandigarh, India.
Correspondence to: Dr Praveen Kumar,
Professor, Department of Pediatrics, Advanced Pediatrics Centre, Post
Graduate Institute of Medical Education and Research, Chandigarh, India.
Email: [email protected]
Received: October 29, 2014;
Initial review: December 05, 2014;
Accepted: May 30, 2015.
(CTRI/2012/03/002530)
|
Objective: To evaluate the efficacy of pre-exchange transfusion
albumin priming in neonates with non-hemolytic hyperbilirubinemia.
Design: Single center, randomized controlled
trial.
Setting: Level III Neonatal unit.
Participants: Fifty healthy term and late preterm
neonates with non-hemolytic hyperbilirubinemia requiring exchange
transfusion.
Interventions: 5 mL/kg of either 20% human
albumin (n=23) or 0.9% saline (n=27) infusion one hour
prior to exchange transfusion.
Main outcome measure: Post-exchange transfusion
phototherapy duration.
Results: The post-exchange transfusion
phototherapy duration was not different between albumin and saline
groups [Median (IQR): 29 (24-48) h vs. 33 (24-43) h; P=0.76].
The total amount of bilirubin removed during exchange transfusion was
also similar [Median (IQR): 34 (28-46) mg vs. 33 (27-38) mg; P=0.46].
Serial changes in total serum bilirubin following exchange transfusion
and need for repeat exchange transfusion were comparable between the
groups.
Conclusions: In healthy late preterm and term
neonates with non-hemolytic hyperbilirubinemia, priming with 1 g/kg of
20% albumin prior to exchange transfusion is not superior to equi-volume
0.9% saline in reducing post- exchange transfusion phototherapy duration
or amount of bilirubin mass removed.
Key words: Exchange transfusion, Jaundice, Neonate,
Phototherapy.
|
|
E
xchange transfusion (ET) is indicated in neonatal
hyperbilirubinemia when other standard therapeutic modalities like
phototherapy (PT) have failed and the risk of acute bilirubin
encephalopathy is high [1]. The efficacy of ET depends on many factors
such as initial total serum bilirubin (TSB), presence of hemolysis,
volume, speed, route and method of ET, size of aliquots, and albumin
concentration in infant’s plasma and donor blood [2]. Albumin within
intravascular space decreases the level of unbound bilirubin in plasma;
to maintain equilibrium between intravascular and extravascular
compartments, unbound tissue bilirubin moves into plasma, and may be
removed during ET. By increasing albumin concentration in plasma,
efficacy of ET may improve. Studies evaluating the efficacy of albumin
for ET in terms of bilirubin removal and its clinical consequences have
shown variable results [3-11]. We conducted this trial to evaluate the
efficacy of pre-ET albumin priming in healthy term and late preterm
neonates with significant non-hemolytic hyperbilirubinemia.
Methods
This randomized controlled trial was conducted in a
tertiary care neonatal unit in Northern India between November 2011 and
January 2013. Healthy (on oral feeds, neurologically normal and
physiologically normal vital parameters) term and late preterm neonates
who were to undergo ET for unconjugated hyperbilirubinemia as per the
decision of the treating team were considered eligible for enrolment.
Infants who were small for gestational age (SGA) or had evidence of
acute bilirubin encephalopathy [12], hemolysis [13], congestive cardiac
failure, hydrops, hematocrit <21% or major congenital malformations,
were excluded. Enrolled infants were randomized into two groups based on
a web-generated random number sequence [14]. Allocation was concealed in
opaque, sealed envelopes which were kept with a person not involved in
any other aspect of the study. Separate personnel, in a separate room
away from patient care area, prepared the study drug. Blinding was
ensured by using special black colored, completely opaque syringes
(Original PerfusorSpritze – OPS 50 mL leur lock, B. Braun Germany) and
brown colored opaque extension intravenous tubing (Lectrogaurd, Vygon,
France) for loading and administration of study drugs. Neonates
randomized to the albumin-primed group received an infusion of 20% human
albumin (Human Albumin 20%, Biotest Pharma, Germany) in a dose of 1 g/kg
(5 mL/kg) over one hour prior to ET. Those randomized to control arm
received 0.9% saline at 5 mL/kg over one hour prior to ET.
Investigators, members of treating team and laboratory technicians
remained masked to the intervention. After enrollment, blood samples
were collected for determining TSB and serum albumin just prior to the
start of the drug infusion. Study participants were closely monitored
for adverse effects such as signs of fluid overload and anaphylactic
reactions. All neonates received phototherapy as soon as
hyperbilirubinemia was diagnosed and continued to receive phototherapy
while study drug was being administered.
ET was followed immediately after the end of
albumin/saline infusion. In the first aliquot of blood drawn from the
infant, TSB, serum albumin and G6PD were measured. A double volume ET
(160 mL/Kg) was done in all cases. The donor blood used for ET was
collected not before five days of ET. During the process of ET, the
blood drawn out (waste blood) was collected in a graduated conical glass
flask. The flask was pre-heparinized by the addition of 2500 units of
heparin before the start of the procedure. To further prevent clotting,
a thin glass rod was kept inserted in the flask to intermittently stir
the collected blood. The total volume of waste blood was measured and
its hematocrit and TSB was determined. All infants were started on
phototherapy and TSB was monitored immediately after the completion of
the ET, and at 2, 6, 12, and 24 hours following ET. Double surface,
standard–length Fluorescent Tube light (STL, 20W/52, Phillips India)
system was used to provide phototherapy in both the groups. Before
starting phototherapy, the irradiance was checked by a photoradiometer
(Fluoro-lite 451®, Minolta/Air Shields, USA). An irradiance of at least
15 µW/nm/cm 2 was maintained
at all times and lamps were replaced whenever necessary. The babies were
placed as close as possible to light source. All attempts were made to
ensure that maximum surface area of baby was exposed to the light
source. Phototherapy was discontinued when 2 TSB values at least 4 hours
apart were 2 mg/dL or more below the phototherapy threshold for that
postnatal age [15]. American Academy of Pediatrics charts adapted as per
the unit protocol were followed for phototherapy and ET thresholds in
neonates ³35
weeks gestation; for neonates <35 weeks, birthweight-based guidelines
were used [15]. Infants in both the groups received intravenous fluid
supplements at presentation, as per unit policy [16,17]. Written
informed consent from one of the parents was obtained before enrolment.
Institutional ethics committee approved the protocol.
Primary outcome was post-exchange duration of
phototherapy. Total mass of bilirubin removed, need for repeat ET,
change in TSB level immediately post-ET, and serial change in TSB levels
at 2, 6, 12, 24 hrs post-ET were secondary outcomes. The post-exchange
duration of phototherapy was calculated from the time of finishing ET to
stopping phototherapy following ET. The total mass of bilirubin removed
during ET (mg) was calculated by standard formula [8]. TSB was measured
by direct spectrophotometry (Twin Beam Micro-bilimeter, Ginevri, Italy).
Bromocresol purple (BCP) dye binding method [18] was utilized for
measuring albumin in serum with Dimension clinical chemistry system
[Siemens Dimension R x L Max,
Siemens Healthcare diagnostics. Atlanta, USA; CV=1.7%]. G6PD screen was
done by Fluorescent spot test.
Sample size and statistical analysis: To detect a
25% decrease in post-ET phototherapy duration in albumin-priming group
in comparison to non-priming group, with a two-tailed
a of 0.05 and power
of 80%, 21 infants per group were required to be enrolled. To account
for attrition, it was decided to enroll a total of 50 infants.
Data entry and analysis was done using statistical software packages
IBM-SPSS v.20 (SPSS Inc. Chicago, IL, USA) and Microsoft Excel. Mann
Whitney U test was applied for primary outcome, change in TSB level
post-ET as compared to pre ET levels and total mass of bilirubin
removed. Unpaired sample Student t test was applied for mass of
bilirubin removed/kg body weight (birth weight). Serial changes in TSB
level post-ET were compared using Repeated Measures ANOVA (analysis of
variance). An Intention to Treat (ITT) analysis was done and a P
value of <0.05 was considered significant.
Results
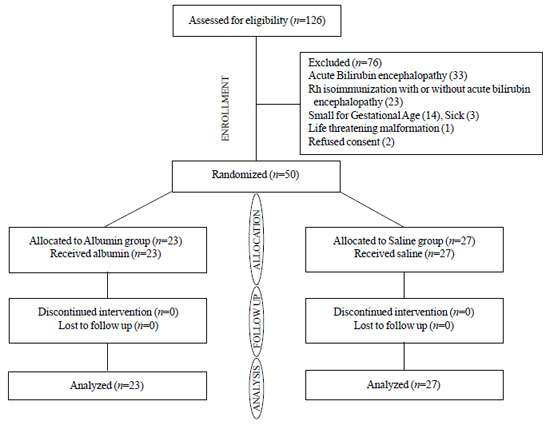
Of the total 50 infants enrolled, ET was done in all,
except one in albumin group, in whom TSB decreased below the exchange
cut-off level following albumin infusion (Fig.1). The
baseline demographic characteristics and factors that could affect
post-ET TSB levels were similar in both groups (Table I
and II).
 |
|
Fig.1 CONSORT diagram of flow of
patients in the trial.
|
TABLE I Baseline Demographic and Laboratory Characteristics
|
Characteristics |
Albumin
|
Saline
|
|
group (n=23) |
group (n=27) |
|
Gestational age (wk) |
38.2 (1.5) |
37.7 (1.7) |
|
Gestational age 34 – 36 wk |
3 (13)** |
5 (19)** |
|
> 37 wk
|
20 (87) |
22 (81)
|
|
Birth weight (g) |
2952 (382) |
2926 (626) |
|
Breastfeeding |
22 (96)** |
19 (70)** |
|
Age of onset of jaundice (h) |
88.2 (36.4) |
83.2 (39.6) |
|
Age at admission (h) |
119.7 (59.3) |
107.1 (42.1) |
|
TSB at admission (mg/dL) |
25.5 (3.6) |
26.1 (4.4) |
|
Pre–ET PT duration (h ) |
19 (12, 27)* |
20 (12, 26)* |
|
TSB prior to ET (mg/dL) |
25.6 (3.6) |
24.8 (3.9) |
|
G6PD deficient |
9 (39)** |
8 (30)** |
|
Serum albumin (g/dL) |
2.8 (0.4) |
2.8 (0.5) |
|
Bilirubin: Albumin ratio#
|
9.1 (1.9) |
9.0 (1.5) |
|
Baseline hematocrit (%) |
46 (7.9) |
50 (6.5) |
|
TSB: total serum bilirubin, ET: exchange transfusion, G6PD:
glucose 6 phosphate dehydrogenase, PT: phototherapy. All values
in mean (SD) except *Median (IQR), **number (%); #in mg: g per
dL. |
TABLE II Effect of Pre-exchange transfusion fluid Infusion on Serum Bilirubin and Albumin Levels
|
Characteristics |
Albumin group |
Saline group |
P |
|
(N=23) |
(N=27) |
|
|
Postinfusion TSB (mg/dL) |
24.2(4.8) |
22.5 (3.9) |
0.16 |
|
Change in TSB (mg/dL). |
-1.4(-3.3, 0.7 )# |
-2.3 (1.2, 3.4) # |
0.36 |
|
Post- infusion serum albumin (g/dL) |
3.4 (0.6) |
2.7 (0.4) |
<0.001 |
|
End of drug infusion to ET interval (min) |
40 (20, 60)* |
30 (15, 60)* |
0.32 |
|
Post – ET hematocrit (%) |
49 (4) |
48 (4) |
0.57 |
|
ET: blood exchange transfusion, TSB: total serum
bilirubin. Values in Mean (SD), *Median (IQR) or #mean
difference (95% CI) |
 |
|
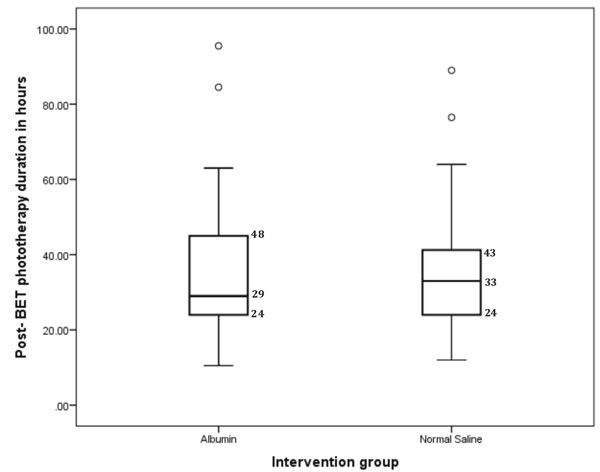
Fig. 2 Box and Whisker plot of post
exchange transfusion phototherapy duration.
|
Forty-seven (94%) infants received intravenous fluid
supplementation prior to intervention as per unit protocol. Volume, rate
and type of the supplemental fluid received were similar in both groups.
Duration of post- ET phototherapy was similar in the two groups (Fig.
2, Table III). Total mass of bilirubin removed, bilirubin
removed per kg body weight (birth weight), need for repeat ET and fall
in TSB immediate post-ET were all similar in both groups (Table
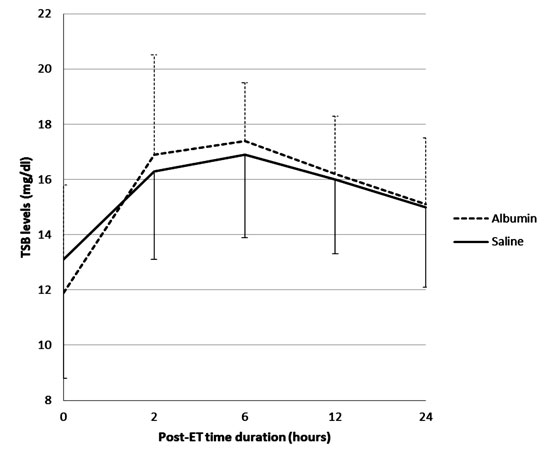
III). Serial change in TSB levels post-ET at different time
intervals was not statistically different between both the groups (Fig.
3). G6PD was found to be deficient in five donor blood samples (2 in
albumin group and 3 in saline group). A subgroup analysis of these cases
did not show any difference in primary outcome between study groups.
TABLE III Comparison of Outcome Between Intervention and Control Groups
|
Characteristics |
Albumin group; n=23 |
Saline group; n=27 |
P |
|
Duration of post-ET phototheraphy (h)
|
29 (24, 48)* |
33 (24, 43)* |
0.76 |
|
Total mass of bilirubin removed during ET (mg) |
34 (28-46)* |
33 (27-38)* |
0.46 |
|
Bilirubin removed/kg birth weight (mg/kg) |
12.5 (3.6) |
12.1 (3.4) |
0.69 |
|
TSB at the end of ET (mg/dL) |
11.9 (3.9) |
13.1 (4.3) |
0.31 |
|
Maximum TSB post- ET (mg/dL) |
18.5 (2.8) |
17.9 (2.9) |
0.50 |
|
Hours post- ET maximum TSB
|
6 (2-12)* |
6 (2-12)* |
0.50 |
|
Need for second ET |
2 (9) # |
2 (7.5) # |
1.00 |
|
ET:exchange transfusion, TSB: total serum bilirubin. All
values are represented as mean (SD) except *Median (IQR)and
#number (%). |
 |
|
Fig. 3 Serial changes in total serum
bilirubin level following exchange transfusion.
|
Discussion
In this randomized controlled trial of pre-ET priming
with 1 g/kg of 20% albumin in term and late preterm infants with
non-hemolytic hyperbilirubinemia, we did not observe a significant
reduction in the post-ET phototherapy duration or an increase in
bilirubin removal following ET. The need for a second ET was not
different between the groups.
TwTwo important limitations in the current study are
intravenous fluids supplementation in majority of the study subjects
before intervention, and possibly lesser albumin dose for priming as the
study neonates had relatively lower albumin levels at baseline.
Intravenous fluid supplementation has been hypothesized to cause a
faster drop in TSB levels due to the effects on volume expansion and
glomerular filtration rate; infants in both the groups received IV
fluids as part of the unit protocol based on previous published studies
[16,17]. The effect of IV fluid supplementation on bilirubin albumin
binding and movement of bilirubin across the capillaries, if any, is not
known. Earlier studies with a beneficial effect of albumin priming had a
higher baseline serum albumin level (3-3.5 g/dL), and a post-priming
albumin level of >5g/dL [4,5,7,8]. A higher serum albumin level may
provide more binding sites for bilirubin and might facilitate the
diffusion of bilirubin into intravascular space better, but safety and
efficacy of high doses need to be demonstrated in our population.
Few studies showed increased bilirubin removal
following priming with 1g/kg of albumin 1-4 hours prior to ET as
compared to simple ET [3-6], whereas others could not demonstrate a
similar effect [7-10]. Shahian and Moslehi [4] demonstrated a
significant reduction in mean TSB levels at 6 and 12 hours after ET as
well as duration of phototherapy in the albumin priming group in
comparison to the control group. The mechanism underlying the benefit
was not reported in their study as well as by a similar study in low
birth weight infants [3], as the amount of bilirubin removed was not
measured in both the studies. Apart from its bilirubin binding
properties, albumin infusion also causes volume expansion [7], which
could independently lead to a reduction in the bilirubin levels after
administration. To prove the hypothesis that albumin priming would
increase the bilirubin removal through its bilirubin-binding properties,
the confounding effect of the volume expansion property of albumin
infusion has to be counter-balanced by using a mother fluid. Most
previous studies did not use any comparator fluid in control group, or
used fluid with a much lower sodium content [3].
We conclude that priming with 1 g/kg of 20% human
albumin in comparison to equi-volume 0.9% saline does not result in an
increase in efficacy of exchange transfusion in healthy term and late
preterm infants with significant non-hemolytic hyperbilirubinemia.
Future studies should evaluate larger doses of albumin, and the effect
of this intervention in hyperbilirubinemia associated with hemolysis.
|
What is Already Known?
• Albumin binds to intravascular bilirubin
and helps draw bilirubin from extra- to intra-vascular space.
What This Study Adds?
• Albumin priming before exchange transfusion does not
increase its efficacy in comparison to priming with equal volume
of 0.9% saline.
|
References
1. Johnson LH, Bhutani VK, Brown AK. System-based
approach to management of neonatal jaundice and prevention of
kernicterus. J Pediatr. 2002;140:396-403
2. Thayyil S, Milligan DW. Single versus double
volume exchange transfusion in jaundiced newborn infants. Cochrane
Database Syst Rev. 2006;4:CD004592.
3. Mitra S, Samanta M, Sarkar M, De AK, Chatterjee S.
Pre-exchange 5% Albumin infusion in low birth weight neonates with
intensive phototherapy failure - a randomized controlled trial. J Trop
Pediatr. 2011;57: 217-21.
4. Shahian M, Moslehi MA. Effect of albumin
administration prior to exchange transfusion in term neonates with
hyperbilirubinemia - A randomized controlled trial. Indian Pediatr.
2010;47:241-244.
5. Odell GB, Cohen SN, Gordes EH. Administration of
albumin in the management of hyperbilirubinemia by exchange
transfusions. Pediatrics. 1962;30:613-21.
6. Kitchen WH, Krieger VI, Smith MA. Human albumin in
exchange transfusion. A quantitative study of the influence of added
human albumin on bilirubin removal. J Pediatr. 1960;57:876-83.
7. Tsao YC, Yu VY. Albumin in management of neonatal
hyperbilirubinemia. Arch Dis Child. 1972;47:250-6.
8. Comley A, Wood B. Albumin administration in
exchange transfusion for hyperbilirubinemia. Arch Dis Child.
1968;43:151-4.
9. Chan G, Schiff D. Variance in albumin loading in
exchange transfusions. J Pediatr. 1976;88:609-13.
10. Ruys JH, Van Gelderen HH. Administration of
albumin in exchange transfusion. J Pediatr. 1962;61:413-7.
11. Wood B, Comley A, Sherwell J. Effect of
additional albumin administration during exchange transfusion on plasma
albumin-binding capacity. Arch Dis Child. 1970;145:59-62.
12. Johnson L, Brown AK, Bhutani V. Bind. A clinical
score for bilirubin induced neurologic dysfunction in newborns. Pediatr
Suppl. 1999;104:746-7.
13. Mishra S, Agarwal R, Deorari AK, Paul VK.
Jaundice in the newborns. Indian J Pediatr. 2008;75:157-63.
14. Variable Block Randomization Software, Available
from: http://www.randomization.com. Accessed April 1, 2015
15. American Academy of Pediatrics. Provisional
Committee for Quality Improvement and Subcommittee on Hyperbilirubinemia.
Management of hyperbilirubinemia in the newborn infant 35 or more weeks
of gestation. Pediatrics. 2004;114:297-316.
16. Saini SS, Kumar P, Balasubramanium K, Mehta S.
Fluid supplementation in hyperbilirubinemia. Indian J Pediatr.
2011;78:1096-9.
17. Mehta S, Kumar P, Narang A. A randomized
controlled trial of fluid supplementation in term neonates with severe
hyperbilirubinemia. J Pediatr. 2005;147:781-5.
18. Lasky FD, Li ZM, Shaver DD, Savory J, Savory MG,
Willey DG, et al. Evaluation of a bromocresol purple method for
the determination of albumin adapted to the DuPont ACA discrete clinical
analyzer. Clin Biochem. 1985;18:290-6.
|
|
|
 |
|

