|
|
|
Indian Pediatr 2015;52: 759-762 |
 |
Outcome of Prevention of Parent-to-Child
Transmission of HIV in an Urban Population in Southern India
|
|
Subramani Seenivasan, Natarajan
Vaitheeswaran,Viswanathan Seetha, *Selvaraj Anbalagan,
*Ramesh Karunaianantham and
*Soumya Swaminathan
From Department of Pediatrics, Government Kilpauk
Medical College, and *National Institute for Research in Tuberculosis
(formerly Tuberculosis Research Centre); Chennai, India.
Correspondence to: Dr S Seenivasan, 28, Indra Nagar,
Walajah Road, Sholinghur, Vellore, Tamilnadu 631 102, India. Email:
[email protected]
Received: January 01, 2014;
Initial review: January 02, 2014;
Accepted: July 15, 2015.
|
Objective: To analyze the outcomes of Prevention of Parent to Child
Transmission (PPTCT) of HIV program in an urban Southern Indian setting.
Design: Observational study.
Setting: Anti-retroviral Therapy (ART) Centers/
Integrated Counseling and Testing Centers (ICTC) at four government
Obstetrics Institutes in an urban area.
Participants: 100 HIV-positive pregnant women and
their infants delivered in the study centers.
Methods: Triple drug ART to HIV-positive pregnant
women was started for maternal indications only. Rest of the pregnant
women were given single dose Nevirapine (200 mg) at the onset of labor.
All infants were given single dose Nevirapine (2 mg/kg) prophylaxis,
according to National AIDS Control Organization guidelines. Mothers were
counseled regarding breastfeeding and artificial feeding, and the choice
was left to them. Whole blood HIV 1 DNA PCR was done for all infants at
6 weeks of life. A second PCR was done at 6 months or 6 weeks after
stopping breastfeeds. PCR-positive infants were started on ART, and were
followed-up till18 months of life.
Results: Four infants were PCR-positive for HIV.
All of them were breastfed. They were born to mothers of HIV stage 1 or
2 who were not on ART as CD4 counts were >350 cells/mm3. Among the
mothers in Stage 3 or 4 or CD4 count <200 cells/mm3 and on ART, none of
the infants was HIV-positive. The cumulative HIV-free survival at 18
months was 94%.
Conclusion: Parent-to-child transmission rate in
HIV was low with the currently used strategies . Triple drug ART to
mother reduces mother-to-child transmission despite advanced maternal
stage or low CD4 counts.
Keywords: HIV-1 DNA PCR, HIV programme, PPTCT.
|
|
T
hough children represent only 6% of the
HIV-infected population, they contribute to one-sixth of HIV-deaths [1].
More than 95% of HIV infections in children are due to vertical
transmission [2]. Deaths due to HIV in children can be reduced through
effective implementation of Prevention of Parent-to-Child Transmission
(PPTCT) program, and by using antiretroviral therapy in HIV-infected
children. When this study was started, National AIDS Control
Organization (NACO) recommended single dose Nevirapine prophylaxis to
both mother and baby with an anticipated reduction of mother-to-child
transmission rate to 10-20% [1].
This study was undertaken to analyze the outcomes of
PPTCT services in an urban population in Southern India, and to study
the factors influencing vertical transmission of HIV.
Methods
The study was undertaken in the Antiretroviral
Therapy (ART) center and Integrated Counseling and Testing Center (ICTC)
at four government Obstetric Institutes in Chennai, India from January
2009 to Febuary 2012 (including 18 months follow up). All pregnant women
in the study setting from January 2009 to August 2010 were screened for
HIV by ELISA test. HIV-positive pregnant women referred from distant
places for institutional delivery, and those who were unlikely to be
followed for 18 months for any reasons, were excluded. The study was
conducted after the Institute’s ethical approval and informed written
consent of the parents.
Those tested positive for HIV were classified into
four clinical stages according to the WHO guidelines [3]. CD4 counts
were done in all HIV-positive women. Triple drug ART (Zidovudine,
Lamivudine and Nevirapine) was started in pregnant women who were in WHO
clinical stage 4, stage 3 with CD4 <350 cells/mm 3,
and those with stage 1 and stage 2 with a CD4 count of <200 cells/mm3.
Single dose (200 mg) Nevirapine was given to all the other HIV-positive
pregnant women at the onset of labor and to the neonates (2 mg/kg) soon
after delivery [1]. All women were counseled regarding breastfeeding and
replacement feeding (undiluted cow milk or formula feed), and the choice
was left to them. If a woman chose to breastfeed, exclusive
breastfeeding was advised up to 6 months and to switch over to
replacement feeds. Thereafter mixed feeding was not advised. Replacement
feeding in the first 6 months was given only if it was Acceptable,
Feasible, Affordable, Sustainable and Safe (AFASS) [1,4]. A child was
defined as ‘breastfed’ if he/she was breastfed for anytime from birth to
six months. A child was defined as non-breastfed if he/she was not at
all breastfed after delivery. No child from both the groups was
breastfed after six months. Infants of all HIV-positive mothers were
followed up for 18 months.
Three mL of blood was collected in vacutainer
containing EDTA. DNA PCR testing was performed using Amplicor HIV-1 DNA
v1.5 kit (Roche molecular Diagnostics, NJ, USA). CD4/CD8 T cell counting
was performed on the BD FACSCalibur flow cytometer. CD4/CD8 percentage
and absolute counting was performed according to the instructions
provided by the manufacturer. Whole blood HIV 1 DNA PCR was done for all
infants at 6 weeks of age. If the infant was PCR- positive, the test was
repeated with a new blood sample as soon as possible, to confirm
diagnosis before disclosure to parents. A second PCR test was done in
all PCR-negative infants at 6 months or 6 weeks after stopping
breastfeeds [1]. A negative test was disclosed only after the second PCR
test was negative, and the child was no longer exposed to breastfeeds.
All children were followed up to 18 months with assessment of
nutritional status and for evidence of any clinical markers of HIV like
oral candidiasis, recurrent respiratory infections, chronic suppurative
otitis media, lymphadenopathy, hepatomegaly, parotid swelling, eczema,
and molluscum contagiosum, at each visit. HIV ELISA was done at 18
months of age. ART was started to HIV-positive babies according to NACO
guidelines [1]. ART regimen was triple drug (Stavudine, Lamivudine,
Nevirapine) as Fixed Dose Combination (FDC) based on weight of infant.
Results
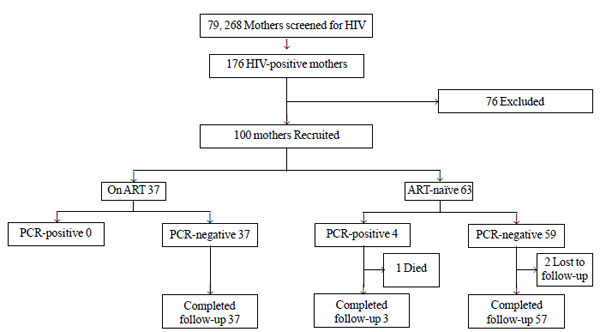
There were 79,268 deliveries during the study period;
176 (0.22%) mothers were HIV ELISA positive (Fig.1).
Seventy-six mothers were excluded because they were referred from
distant secondary-care hospitals for delivery or because they were
unlikely to be followed for 18 months for social reasons.
 |
|
Fig.1 Study population and follow-up.
|
One hundred included women were divided into three
groups based on CD4 counts <200 (n=5), 200 to 350 (n=19)
and >350 (n=76). HIV clinical staging in pregnant women was Stage
1 (n=33), Stage 2 (n= 55), Stage 3 (n=10) and Stage
4 (n= 2). Thirty seven mothers were on triple drug ART. The
median gestational age of starting ART was 16 (IQR 0, 28) weeks. The
mean (SD) birth weight of the infants was 2710 (344) g. Twelve were born
preterm. Forty-two infants were born by vaginal delivery and 58 by
Caesarean section.
All 100 neonates received single dose Nevirapine soon
after delivery. Sixty mothers chose to breastfeed. Among the breastfed,
only three infants were breastfed for the entire 6 months; others were
breastfed for variable periods from 1 month to 5 months and were
switched to replacement feeds. The median (IQR) duration of breast
feeding was 3 (2, 3.5) months. Forty mothers chose replacement feeds
(artificial formula or undiluted cow’s milk) from day 1 of life. There
was no mixed feeding.
Three infants were HIV DNA PCR-positive at 6 weeks
and 97 were negative. Among babies tested negative, the second sample at
6 months yielded one more positive result. The details of PCR-positive
infants are given in Table I. Two infants were born to
mother with stage 1 disease and other two with stage 2 disease. No child
born to mothers with stage 3 or 4 who were on ART developed HIV
positivity. CD4 counts of mothers of four positive infants were more
than 350/mm 3. All the four
positive infants were referred to Pediatric ART Center at Institute of
Child Health. One child died of broncho-pneumonia at 6 month of age; his
CD4 count was 1880 cells/mm3
(27%). Two of the PCR-negative babies were lost to follow up.
TABLE I Maternal History and Profile of HIV-1 DNA PCR-positive Infants
|
A |
B |
C |
D |
|
Maternal history |
|
Age (y) |
23 |
31 |
30 |
27 |
|
Weight (Kg) |
45 |
66 |
57 |
56 |
|
Gravida |
Primi |
Primi |
G4P3L3A0 |
G2P1L1A0 |
|
CD4 Count (Cell/mm3) |
467 |
834 |
890 |
491 |
|
HIV Stage |
1 |
1 |
2 |
2 |
|
ART status |
No |
No |
No |
No |
|
Spouse HIV status |
Positive |
Positive |
Positive |
Positive |
|
Bleeding PV |
Yes |
No |
No |
Yes |
|
PROM >4 hours |
No |
No |
No |
No |
|
Infant demographics |
|
Gender |
Male |
Female |
Male |
Female |
|
Birth Weight (g) |
2500 |
2900 |
2250 |
3000 |
|
Mode of delivery |
LSCS |
LSCS |
Vaginal |
Vaginal |
|
Nevirapine |
Yes |
Yes |
Yes |
Yes |
|
*Duration of breastfeeding |
3 mo |
4 mo |
2 mo |
4 mo |
|
HIV stage |
Stage 1 |
Stage 1 |
Stage 1 |
Stage 1 |
|
CD4 Count (cells/mm3) |
3411 |
2718 |
1880 |
1898 |
|
CD4 % |
47 % |
41 % |
27 % |
32 % |
|
CD8 Count (cells/mm3) |
1161 |
1859 |
2437 |
2140 |
|
CD8 % |
16 |
26 |
35 |
36 |
|
CD4 / CD8 Ratio |
2.94 |
1.46 |
0.77 |
0.89 |
ART- Anti Retroviral Therapy, LSCS- Lower Segmental Caesarean Section, PROM- Prolonged Rupture of Membrane.
*All infants were breastfed exclusively.
|
Discussion
The overall parent-to-child transmission rate in this
study was 4%; it was 6.3% with single dose Nevirapine alone. This
transmission rate was less than the expected 10 to 20% in a pilot study
done by NACO [1]. However, it was similar to a study from Chennai with
an overall transmission rate of 8.3% from a sample of 218 dried blood
spot DNA PCR [5]. Others have shown even higher transmission rates
[6,7]. Marinda, et al. [8] showed HIV-positive mothers with more
advanced disease are more likely to infect their infants. However, in
our study, all 4 PCR-positive babies were born to mothers who were in
stage 1 or stage 2, and whose CD4 count was >350 cells/mm3.
Marazzi, et al. [9] showed a transmission rate of 50.6% from
mothers with CD4 count >350 cells/mm3
but these women were not on ART. Ugochukwu, et al. [10] found
lower transmission rates when both mother and baby were on prophylaxis.
This shows that triple drug ART reduces the transmission rate even in
advanced maternal disease or in the presence of low CD4 counts.
Moreover, recent guidelines and several studies recommend triple drug
regimens to prevent parent-to-child transmission of HIV [11-13]. Single
dose Nevirapine may also be associated with increased risk of resistance
[14].
Though transmission rates were 6.7% and 0% in
breastfed and non-breastfed groups, respectively; we do not attribute
PCR positivity to breastfeeding alone as three of the four infants were
PCR-positive at 6 weeks of life. This was probably due to intrapartum
transmission. Palombi, et al. [15] showed a transmission rate of
<2% with alternatives to breastfeeding without an increase in mortality
in non-breastfed group. The cumulative HIV-free survival at 18 months in
our study was similar to that reported in an earlier study [9].
The limitations of our study were small sample size,
and that our study population mostly belonged to lower and lower-middle
class which may not be representative of the entire population.
We conclude that the overall parent-to-child
transmission rate of HIV is low when the pregnant women receive ART, and
single dose Nevirapine is given to the infants, simultaneously avoiding
mixed feeding.
Acknowledgement: Project Director, Tamilnadu
State AIDS Control Society.
Contributors: SS, SS: conceived and designed the
study; SS, NV: followed up with the patients, conducted clinical
assessment, collected data; AS, RK: conducted and laboratory tests, and
data analysis; SS: drafted the manuscript; VS, SS: critical revision of
the manuscript for important intellectual content. All authors approved
the final version of manuscript.
Funding: Indian Council of Medical Research;
Competing interests: None stated.
|
What is Already Known?
• Parent-to-child transmission of HIV occurs
with advanced maternal disease and low CD4 counts.
What This Study Adds?
• Most of vertical HIV transmissions occur
when mothers are not on triple drug ART.
• Parent-to-child transmission rate with
single dose Nevirapine prophylaxis to mother and baby is low.
|
References
1. Shah NK, Mamta M, Shah I, Deepak U, Lodha R, Pensi
T, et al. Guidelines for HIV Care and Treatment in Infants and
Children, 1st ed. New Delhi: National AIDS Control Organisation and
Indian Academy of Pediatrics, 2006. p. 3-90.
2. Shah NK. Epidemiology and Trend of HIV in India.
In: Shah I, Shah NK, Manglani M, editors. IAP Speciality Series
on Pediatric HIV. 1st ed. Mumbai: Indian Academy of Pediatrics,
2006. p. 11-69.
3. . World Health Organization, 2010. Guidelines on
HIV Prevention, Treatment and Care. Available from: www.
who.int/hiv/pub/en/2010guidelines. Accessed November 10, 2014.
4. UNAIDS. Preventing mothers from dying and babies
from becoming infected by HIV. Available from: http:www.
unaids.org/en/media/unaids/contest/documents/unaids
publication/2010/2010103. Accessed November 10, 2014.
5. Jacob SM, Anitha D, Vishwanath R, Parameshwari S,
Samuel NM. The use of dried blood spots on filter paper for the
diagnosis of HIV-1 in infants born to HIV seropositive women. Indian J
Med Microbiol. 2008;26:71-4.
6. Gbadegesin A, Adenibuyan OA, Adegbesan MA, Salu
OB, Omilabu SA. Efficacy of HIV PCR techniques to diagnose HIV in
infants born to HIV infected mothers at LASUTH. Nig Q J Hosp Med.
2010;20:129-32.
7. Shah I. Efficacy of HIV PCR techniques to diagnose
HIV in infants born to HIV infected mothers – an Indian perspective. J
Assoc Physicians India. 2006;54:197-9.
8. Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo
KJ, Piwoz EG, et al. Child mortality according to maternal and
infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519-26.
9. Marazzi MC, Liotta G, Nielsen-Saines K, Haswell J,
Magid NA, Buonomo E, et al. Extended antenatal antiretroviral use
correlates with improved infant outcomes throughout the first year of
life. AIDS. 2010;24:2819-26.
10. Ugochukwu EF, Kanu SO. Early infant diagnosis of
HIV infection in southeastern Nigeria: prevalence of HIV infection among
HIV-exposed babies. West Afr J Med. 2010;29:3-7.
11. World Health Organization. Consolidated
Guidelines on the Use of Antiretroviral Drugs for Treating and
Preventing HIV Infection. Available from:
www.who.int/hiv/pub/guidelines/arv2013/download/en. Accessed January
7, 2015.
12. National AIDS Control Organization. Updated
Guidelines for Prevention of Parent to Child Transmission of HIV using
Multi Drug Anti-retroviral Regimen in India. Available from:
www.naco.gov.in/upload/NACP-IV/18022014 BSD/
National_Guidelines_for_PPTCT.pdf. Accessed January 7, 2015.
13. Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C,
Misore A, et al. Triple-antiretroviral prophylaxis to prevent
mother-to-child HIV transmission through breastfeeding-the Kisumu
Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015.
14. Micek MA, Blanco AJ, Beck IA, Dross S, Matunha L,
Montoya P, et al. Nevirapine resistance by timing of HIV type 1
infection in infants treated with single-dose nevirapine. Clin Infect
Dis. 2010;50:1405-14.
15. Palombi L, Marazzi MC, Voetberg A, Magid NA.
Treatment acceleration program and the experience of the DREAM program
in prevention of mother-to-child transmission of HIV. AIDS. 2007;
21:S65-71.
|
|
|
 |
|

