|
|
|
Indian Pediatr 2012;49:
717-720 |
 |
Predictors of Significant Jaundice in Late
Preterm Infants
|
|
K Radha Lavanya, Ashish Jaiswal, Pramod Reddy and Srinivas Murki
From Fernandez Hospital, Hyderabad, Andhra Pradesh, India.
Correspondence: Dr Srinivas Murki, Consultant Neonatologist,
Fernandez Hospital, Hyderabad,
Andhra Pradesh 500 001, India.
Email:
srinivasmurki2001@yahoo.com
Received: June 27, 2011;
Initial review: July 12, 2011;
Accepted: October 24, 2011 .
Published online: March 30, 2012.
SII:S097475591100553-1
|
Objectives: To study (i) the incidence and course of
jaundice, and (ii) the predictors of ‘significant jaundice’ in
late preterm infants.
Design: Prospective analytical study.
Setting: Urban perinatal center.
Patients: Inborn late preterm infants (post
menstrual age of 34 0/7 to 36 6/7 weeks).
Methods: Infants were followed till day 14 of
life or till onset of significant jaundice. Relevant maternal, perinatal
and neonatal variables were prospectively recorded. Transcutaneous
bilirubin (TcB) was measured in each infant twice daily for the first 48
hours of life.
Outcomes: Significant jaundice defined as
requirement of phototherapy/exchange transfusion as per hour specific
total serum bilirubin (TSB) nomogram of AAP guidelines.
Results: 216 infants were enrolled, of which 123
(57%) had significant jaundice. 36% of the jaundiced infants had TSB
greater than 15 mg/dL. The mean duration of onset of significant
jaundice was 61 ± 32 hours. The mean duration of phototherapy was 49 ±
26 hours. Large for gestation, lower gestational age, birth trauma and
previous sibling with jaundice predicted severe jaundice. TcB measured
at 24-48 hrs was a better predictor of ‘significant jaundice with onset
after 48 hrs’ than clinical risk factors.
Conclusion: There is a high incidence of
significant jaundice in late preterm infants. TcB measured at 24-48 hrs
of life better predicts ‘significant jaundice after 48 hours of life’,
in comparison with clinical risk factors.
Key words: Late-preterm, Outcome, Prediction, Significant
jaundice, Transcutaneous bilirubin.
|
|
Jaundice in the newborn is a common cause for
hospital readmission during the first week of postnatal life [1]. Late
preterm gestation has been identified as one of important risk factor
for the development of severe jaundice and kernicterus [2]. Jaundice in
late preterm infants is more prevalent, more pronounced, and more
protracted in nature than in their term counterparts [3]. With most
infants getting discharged at or before 48 hours of life, outpatient
follow-up is needed to identify the infants in whom total serum
bilirubin levels will rise high enough to require treatment. The
American Academy of Pediatrics (AAP) clinical practice guidelines on the
management of neonatal hyperbilirubinemia recommend that all newborn
infants be assessed before discharge for the risk of developing
subsequent severe hyperbilirubinemia [4]. Several recent studies have
looked at ways of predicting the risk of significant post discharge
jaundice by taking measurements before hospital discharge [5-7]. All the
previous studies have evaluated the predictors of jaundice in term and
near term infants, but none exclusively in this high risk group.
Methods
All consecutive inborn late preterm infants between 1 st
February to 31st July, 2009
with post-menstrual age of 34 0/7 to 36 6/7 weeks were eligible for
inclusion. Infants with major malformations and Rh incompatibility were
excluded. Gestational age was assessed from the first trimester
ultrasound or mother’s last menstrual period. The relevant perinatal and
neonatal data were recorded prospectively in a predesigned case
reporting form. A single trained nurse did the TcB measurement in each
infant twice daily (7 AM – 9 AM and 6 PM – 8 PM) for the first 48 hours
of life. All measurements were obtained from the forehead using
transcutaneous bilirubinometer (Bilichek-HHU, Respironics). Whenever the
neonate was clinically jaundiced, or when TcB was >12mg/dL, total serum
bilirubin (TSB) estimation was done. Treatment of jaundice was based on
the TSB and not on the TcB. TSB was estimated from the capillary sample
using spectrophotometer (Unibeam). Significant jaundice was defined as
requirement of phototherapy/exchange transfusion as per hour specific
total serum bilirubin (TSB) nomogram of AAP guidelines. Infants
discharged from the hospital were followed in the outpatient clinic
daily till day 14 of life or till onset of significant jaundice.
Neonates with significant jaundice were started on phototherapy as per
the AAP guidelines [4]. Infants with gestation 34 weeks and SGA infants
were started on phototherapy at TSB levels 1mg/dL less than the
treatment threshold on the AAP charts.
Continuous variables were summarized using mean ± SD
and categorical variables as frequencies and percentages. Based on the
hour of measurement, the TcB measurements were grouped into TcB 0-12
hrs, TcB 13-24 hrs, TcB 25-36 hrs and TcB 37-48 hrs. The clinical risk
factors and the grouped TcB measurements were compared between infants
with and without significant jaundice after 48 hours of life.
Sensitivity, specificity, predictive values and ROC curves were plotted
for all significant clinical variables and median TcB measurements for
the prediction of significant jaundice. Discriminative ability of
predictive variables for the outcome of interest was compared with ROC
curves (area under the curve). P value <0.05 was considered
significant.
The study was approved by the Institute’s ethics
committee and consent was obtained from the parents immediately after
the birth of the child.
Results
Of the 265 late preterm infants born in the hospital
during the study period, 216 (58% males) were enrolled in the study (Fig.
1). Two hundred and thirteen infants were followed till onset of
significant jaundice or till day 14 of life. The mean gestation and the
mean weight of study subjects were 35.42 ±0.75 weeks and 2375 ± 490
grams, respectively. Thirty four (16%), 56 (26.3%) and 123 (57.7%)
infants were of gestation 34, 35 and 36 weeks, respectively, 23 (10.8%)
neonates were small for gestation (SGA) and 15 (7%) were large for
gestational age (LGA). Forty six (21.5 %) infants were born of twin or
triplet gestation. One twenty three (57%) subjects developed significant
jaundice. The incidence of jaundice was significantly higher at 34 (65%)
and 35 weeks (68%) than at 36 weeks (51.2%) (P =0.02). The mean
duration of onset of significant jaundice was 61 ± 32 hours. Three
(1.4%) infants developed significant jaundice within first 24 hours of
life, 61 (26.5%) infants between 25 to 48 hours of life and 59 (28.8%)
after 48 hours of life. The mean duration of phototherapy was 49 ± 26
hours. The mean peak TSB was 14.3 mg/dL ±2.6; 36% of the jaundiced
infants had TSB greater than 15 mg/dl. Among the infants who developed
significant jaundice after 48 hours, the mean age of onset was 81±34
hours, the mean peak bilirubin was 14.7±2.8 mg/dL, and the mean duration
of phototherapy was 41±18 hours.
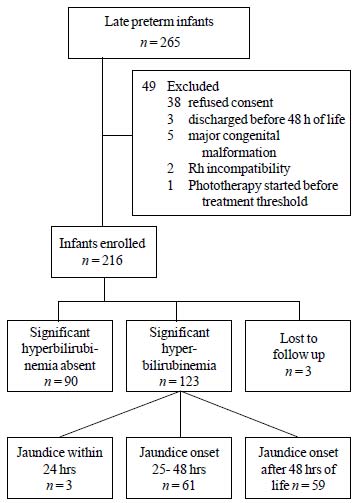 |
|
Fig. 1 Enrollment and follow up of
study subjects.
|
Mother’s blood group O and neonate’s blood group A or
B or AB, birth trauma, history of jaundice in previous sibling, large
for gestation, and gestation 34 or 35 weeks was significantly higher in
neonates who developed significant jaundice (Table I). The
median TcB values were 1.3 mg/dL, 4 mg/dL, 5.9 mg/dL, and 7.5 mg/dL at
0-12 hrs, 13-24 hrs, 25-36 hrs and 37-48 hrs of life, respectively. The
mean TcB (13-24hrs) was significantly higher in infants who developed
significant jaundice between 24 to 48 hours compared with those that
developed jaundice after 48 hours of life (4.9 ±2.3 vs 4.1±1.6
mg/dL) (P =0.03) On comparing the clinical risk factors with TcBs,
the ability to discriminate neonates with and without significant
jaundice was better for grouped median TcBs from 24 to 48 hours i.e. TcB
25-36 hrs (AUC 0.75) and TcB 37-48 hrs (AUC 0.73) (Web
Table I).
TABLE I Risk Factors in Neonates With and Without ‘Significant Jaundice After 48 Hours of Life
|
Variable |
Significant Hyperbilirubinemia |
|
Absent (n=90)
|
Present (n=90) |
|
Birthweight (g)*
|
2420 ± 411 |
2404 ± 609 |
|
Gestation (wks)‡ |
35.42 ± 0.75 |
35.29 ± 0.72 |
|
Males
|
46 (51.1%) |
35 (59.3%) |
|
SGA (Small for gestation)# |
6 (6.7%) |
9 (15.3%) |
|
LGA (Large for gestation)$ |
3 (3.3%) |
11 (18.6%) |
|
OA setting#
|
2 ( 2.22%) |
6 (10.1%) |
|
Maternal oxytocin |
4 (4.4%) |
6 (10.1%) |
|
Sibling jaundice# |
0 |
4 (6.8%) |
|
Birth trauma |
0 |
4 (6.8%) |
|
Exclusive Breastfeeding |
22 (24.4%) |
10 (16.9%) |
|
Stools/day**
|
2 (1-3) |
2 (1-3%) |
|
Meconium passage (in d)** |
2 (1-5) |
2 (1-6%) |
|
Weight loss/d (g)
|
30 ± 27 |
33 ± 22 |
|
NICU admission |
17 (18.8%) |
18 (30.5%) |
|
*Mean±SD, numbers in parenthesis are percentages; #P<0.05;
$P=0.001; ‡P=0.05; significant jaundice defined as requirement
of phototherapy/exchange transfusion as per hour specific total
serum bilirubin (TSB) nomogram of IAP guidelines; **Values in
median (range). |
Discussion
This study is one of the few prospective studies to
evaluate the incidence and course of jaundice in late-preterm infants.
Fifty seven percent of late preterm infants developed significant
jaundice. This highlights a need for the early recognition and screening
of jaundice in this group.
In the only other prospective study comparing near
term and term infants, the incidence of significant jaundice was 25.3%
in near term infants [8]. In a retrospective study performed in a well
infant population, infants of 35 to 36 weeks, 36 to 37 weeks and 37 to
38 weeks gestation were 13.2, 7.7 and 7.2 times more likely,
respectively, to be readmitted to hospital and require phototherapy for
significant jaundice than those of >39 weeks gestation [1]. Similarly in
our study, infants of lower gestation were at higher risk of developing
significant jaundice. The high incidence of significant jaundice in late
preterm infants may be attributed to their inability to handle bilirubin
load, decreased hepatic UDP glucuronyl transferase enzyme activity, and
a slower post natal maturity of hepatic bilirubin uptake [9,10]. In
contrast to the study by Sarici, et al. [9], a higher incidence
and early onset of significant jaundice in our study may be explained by
the inclusion of infants with 34 weeks gestation and by the difference
in definition of significant jaundice.
As demonstrated in other studies evaluating
pre-discharge risk assessment [11], large for gestation, gestational
age, birth trauma and previous sibling with severe jaundice are the
clinical variables significantly associated with significant jaundice in
our study. Pre-discharge TcB as a predictor variable was similar or
sometimes even better than clinical risk factors alone for prediction of
significant jaundice. In the only other prospective cohort study on term
and near term infants [6], combining predischarge TcB measurements with
gestational age (compared with TcB measurement alone) improved the
accuracy of the prediction of a subsequent TSB rising to within 1mg/dl
of the hour specific phototherapy threshold recommended by the AAP.
Considering that 25% to 50% of late preterm infants are at risk for
subsequent jaundice, routine predischarge TcB measurement can help in
predicting infants’ needing delayed discharge and/or early follow up
assessment for neonatal jaundice.
Treatment of late preterm infants with gestation 34
weeks as per APP guidelines and special treatment of SGA infants, are
some the limitations of this study. There are no other published
nomograms validated for our population and for uniformity of management
we used the AAP guidelines.
There is a very high incidence of significant
jaundice in late preterm infants. TcB measured at 24-48 hrs of life
significantly predicts significant jaundice after 48 hours of life,
which may help in identification of neonates requiring delayed discharge
or early follow up assessment for jaundice after hospital discharge.
Contributors: SM designed the study and
supervised the data collection. RL collected and analyzed the data. PG
was involved in study design. All the authors were involved in
preparation of the manuscript.
Funding: None; Competing interests:
None stated.
|
What is Already Known?
• Late preterm infants are at increased risk
of jaundice compared with term infants.
What This Study Adds?
• TcB values greater than 5.9 mg/dL between
24 to 36 hours of life, and >7.5mg/dL between 37 to 48 hours of
life better predict subsequent onset of significant jaundice
than any of the clinical risk factors.
|
References
1. Maisels MJ, Kring E. Length of stay, jaundice, and
hospital readmission. Pediatrics. 1998;101:995-8.
2. Bhutani VK, Johnson LH, Maisels MJ, Newman TB,
Phibbs C, Stark AR, et al. Kernicterus: Epidemiological
strategies for its prevention through systems-based approaches. J
Perinatol. 2004;24:650-62.
3. Billing BH, Cole PG, Lathe GH. Increased plasma
bilirubin in newborn infants in relation to birth weight. BMJ.
1954;2:1263-5.
4. American Academy of Pediatrics, Subcommittee on
Hyperbilirubinemia. Clinical Practice Guideline: Management of
Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation.
Pediatrics. 2004;114:297-316.
5. Stevenson DK, Fanaroff AA, Maisels MJ, Young BWY,
Wong RJ, Vreman HJ, et al. Prediction of hyperbilirubinemia in
near-term and term infants. Pediatrics. 2001;108:31-9.
6. Keren R, Luan X, Freidman S, Saddlemire S, Cnaan
A, Bhutani V. A comparison of alternative risk-assessment strategies for
predicting significant neonatal hyperbilirubinemia in term and near-term
infants. Pediatrics. 2008; 12:170-9.
7. Maisels MJ, DeRidder JM, Kring EA, Balasubramaniam
M. Routine transcutaneous bilirubin measurements combined with clinical
risk factors improve the prediction of subsequent hyperbilirubinemia.
Perinatol. 2009;29:612-7.
8. Sarici SU, Serdar MA, Korkmaz A, Erdem G, Oran O,
Tekinalp G, et al. Incidence, course, and prediction of
hyperbilirubinemia in near-term and term newborns. Pediatrics.
2004;113:775-80.
9. Kaplan M, Muraca M, Vreman HJ, Hammerman C, Vilei
MT, Rubaltelli FF, et al. Neonatal bilirubin production –
conjugation imbalance: effect of glucose-6-phosphate dehydrogenase
deficiency and borderline prematurity. Arch Dis Child Fetal Neonatal Ed.
2005;90:123-7.
10. Kawade N, Onish S. The prenatal and postnatal
development of UDP-glucuronyltransferase activity towards bilirubin and
the effect of premature birth on its activity in the human liver.
Biochem J. 1981;196:257-60.
11. Keren R, Bhutani VK. Predischarge risk assessment
for severe neonatal hyperbilirubinemia. Neo Reviews. 2007;8:68-76.
|
|
|
 |
|

