|
|
|
Indian Pediatr 2011;48:
689-696 |
 |
Evaluation of Phototherapy Devices Used for
Neonatal Hyperbilirubinemia |
|
Sreeram Subramanian, Mari Jeeva Sankar, Ashok K Deorari,
*Thirumurthy Velpandian, †Pradeesh Kannan,
†Gaddam Vijaya Prakash, Ramesh Agarwal and Vinod K
Paul
From the Division of Neonatology, Department of
Pediatrics, and *Division of Ocular Pharmacology, Dr RP Centre for
Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi;
and †Nanophotonics Division, Department of Physics, Indian Institute of
Technology, New Delhi, India.
Correspondence to: Dr Ashok K Deorari, Professor,
Division of Neonatology, Department of Pediatrics, Co-ordinator, WHO-CC
for Training and Research in Newborn Care, All India Institute of Medical
Sciences,
Ansari Nagar, New Delhi 110 029, India.
Email:
ashokdeorari_56@hotmail.com
Received: October 12, 2009;
Initial review: November 4, 2009;
Accepted: August 3, 2010.
Published online 2010 November 30.
PII: S097475590900738-1
|
Objective: To compare phototherapy devices based on
their physical and photo-biological characteristics viz spectral
properties, maximum and mean irradiance, treatable percentage of body
surface area, decay of irradiance over time and in vitro
photoisomerisation of bilirubin.
Design: In vitro experimental study.
Setting: Ocular pharmacy laboratory at a tertiary
care hospital.
Methodology: All the characteristics were measured
at a fixed distance of 35 cm from one compact fluorescent lamp (CFL) and
three light emitting diode (LED) phototherapy devices in a dark room with
an irradiance of <0.1µW/cm2/nm. Estimation of products of in
vitro photoisomerisation was done using liquid chromatography - tandem
mass spectroscopy (LC-MS/MS).
Results: The emission spectral data were comparable
between the phototherapy devices. The devices, however, differed in their
maximum irradiance with the spot and indigenous LED units having the
highest and lowest values, respectively (56.5 and 16.8µW/cm2/nm).
The mean irradiance – measured in 5x5cm grids falling within the
silhouette of a term baby – of the spot and improvised LED devices were
low (26.8µW/cm2/nm and 11.5µW/cm2/nm, respectively)
possibly due to unevenness in the irradiance of light falling within the
silhouette. There was a significant difference in the amount of bilirubin
left after exposure to light over a 2-hour time period (% reduction of
bilirubin) among the four devices (P=0.001); at 120 minutes after
exposure, the amount of bilirubin left was lowest for the CFL (16%) and
spot LED (17%) devices and highest for the indigenous LED unit (41%).
Conclusions: The four phototherapy devices differed
markedly in their physical and photobiological characteristics. Since the
efficacy of a device is dependent not only on the maximum irradiance but
also on the mean irradiance, rate of decay of irradiance, and treatable
surface area of the foot print of light, each phototherapy device should
have these parameters verified and confirmed before being launched for
widespread use.
Key words: Efficacy, Neonate, Jaundice, Phototherapy, Compact
fluorescent tube, Light emitting diode.
|
|
Phototherapy should be regarded as a drug, with an appropriate dose and
duration, used to manage hyperbilirubinemia in neonates. There is no
standardized method for reporting phototherapy dosages in the clinical
practice. The ‘dose’ of phototherapy would depend upon the device
characteristics such as emission spectral data, maximum irradiance, mean
irradiance, treatable percentage of body surface area (BSA), age of the
light source, and possibly the amount of formation of photoisomers from
bilirubin.
Currently, no guidelines are available for measuring
the efficacy of different phototherapy devices used in the country. Bench
studies from the West have shown widely varying efficacy of these devices
[1-4]. One of the few studies from India that evaluated the phototherapy
devices used in different hospitals of a major city found only 31% of the
units to be providing an acceptable level of irradiance (at least 15µW/cm 2/nm)
and a meager 8% of the devices to have the
recommended special blue lights [5].
Neonates with hyperbilirubinemia treated with
suboptimal devices may require prolonged photo-therapy or even exchange
transfusion because of failure of phototherapy. There is a need to
standardize the phototherapy devices so that effective devices are used
for the management of neonatal hyperbilirubinemia. The present study was
designed to evaluate and compare different phototherapy devices and also
to develop standardized methods for evaluation.
Methods
We tested four different phototherapy devices: (i)
Spot LED (Phoenix Medical Systems Pvt Ltd, India); (ii) Indigenous
light emitting diode (LED) (Photolux, SriChakra Scientifics, India); (iii)
Improvised LED (Bilitron Fanem Inc, Brazil); and (iv) Compact
fluorescent lamp (CFL) unit (Phoenix Medical Systems Pvt Ltd, India). The
experiments were conducted in a dark room with irradiance of <0.1 µW/cm 2/nm
and at fixed distance of 35 cm. The devices tested, except for indigenous
LED, were brand new devices. The spot LED device has a high intensity (40
W) LED bulb encased in a cup shaped enclosure fixed on a pedestal. The
indigenous LED device consists of green LED (33 bulbs) arranged in three
center rows and multiple rows of blue LED (176 bulbs) flanking the green
on either side. The improvised LED unit consists of 5 high intensity LED
bulbs mounted on a mobile pedestal. A fan within the unit helps to
dissipate the heat. The CFL phototherapy device consists of six 18 W
double folded (8 inches) special blue CFL encased in a rectangular box
fitted with a light reflector.
Measurement of surface area: A white spacer board
made up of packing material was cut to size 60×30 cm and a white paper
having vertical and horizontal lines forming grid size of 5×5 cm was
pasted on it. Silhouette of a term (gestational age 38 wks) appropriate
for gestational age baby was then traced on the white paper. The surface
area of the silhouette was 780 cm2.
The size of the board was similar to that recommended by International
Electrotechnical Commission (IEC) which defines the "effective surface
area" as the intended treatment surface that is illuminated by the
phototherapy light [6].
Comparison of peak emission spectra: We measured
the peak emission spectra of the lamps of the four devices at
Nanophotonics Division, IIT Delhi, using a portable HR2000CG-UV-NIR
optical spectrum analyzer (Ocean Optics Florida, USA). This high
resolution spectrometer has a detector that covers the 200-1100 nm
wavelength range and interfaces to a personal computer via USB 2.0 port.
The photo-therapy devices were transported to the laboratory and spectral
data were recorded.
Comparison of spectral irradiance: Spectral
irradiance was measured using spectroradiometer (Biliblanket Meter II;
Ohmeda Medical, GE Health care, USA). This instrument is a fixed spectroradiometer
capable of picking up irradiance between 400-520 nm with peak sensitivity
at 450 nm. The measurements were done at the center of the measuring
surface and at four perpendicular peripheral points (at a distance of 15
cm [breadth-wise] and 30 cm [lengthwise] from the center) once a day for
three consecutive days and the average of the three readings was taken.
Comparison of mean irradiance: The spacer board was
placed under the different phototherapy systems and the spectral
irradiance was measured in each of the 5x5cm grid falling within the
silhouette of the baby. The mean irradiance was determined by averaging
the spectral irradiance obtained in each of these grids.
Decay of spectral irradiance: All the phototherapy
devices were switched on and allowed to run continuously for a total
duration of one month. The instruments were connected through a voltage
regulated power source to avoid fluctuations in power supply. Spectral
irradiance was checked daily for a period of one month at the center of
the field and at the four peripheral perpendicular points. The decay of
spectral irradiance over time was calculated.
Treatable body surface area: As it is difficult to
calculate the body surface area of the irregular shaped term baby outline,
we used an indirect method suggested by Vreman, et al. [1].
In vitro quantification of bilirubin and confirmation
of photoisomers: Thermo Finnigan high performance liquid
chromatographic (LC) system (Thermo Electron Corp, Waltham, MA, USA) with
PDA detector controlled by ChromQuest (Ver.4.5) software was used to elute
the analyte. Electron spray ionization technique in positive mode was
applied using Turbo Ionspray source (Applied Biosystems, Foster city, CA,
USA) in a 4000 Q trap tandem mass spectroscopy (MS/MS) (MDS SCIEX, Applied
Biosystems, Foster city, CA, USA). Tandem mass spectroscopy was controlled
using Analyst (Ver.1.4.2) software.
HPLC conditions: For the analytical separation,
hydrophilic interaction chromatography technique was employed using
ZIC-HILIC column (50x4.6mm, 3.5µm particle size; Merck SeQuant AB, Umea,
Sweden).
The samples amounting to 20µL were added to 200µL of
extraction solvent (70% acetonitrile containing 0.1% formic acid)
containing 250ng of homatropine (internal standard) and subjected to
vortex for 1 min and loaded into the HPLC autosampler for analysis.
Samples were injected at the volume of 20µL and each run was optimized for
3 minutes. Serially diluted standards varying from 0.98ng/mL to 250ng/mL
were injected in triplicate and used for quantification. Interday and
Intraday variations for the above standards were found to be within the
limits of CV<10%. The LC-MS/MS data was analyzed using ‘Analyst’ (Ver.
1.4.2).
Confirming the production of photodegraded products
having similar molecular weights as that of bilirubin: The
photoisomerization of bilirubin was analyzed by using standard bilirubin
diluted from the stock solution reaching the concentration of 100ng/mL.
Three 1.2 mL vials (using auto sampler vials of HPLC) containing 1mL of
the methanolic bilirubin solution (concentration of 1 µg/mL) were placed
horizontally on a white background kept at a distance of 35 cm from the
spot LED lamp at the point of maximum irradiance (previously determined by
using fixed spectroradiometer). 10 µL of above solution was aspirated
prior to and after 2, 12, 22, 37, and 67 minutes of exposure to light and
subjected for analysis using LC/MS/MS using the conditions stated above.
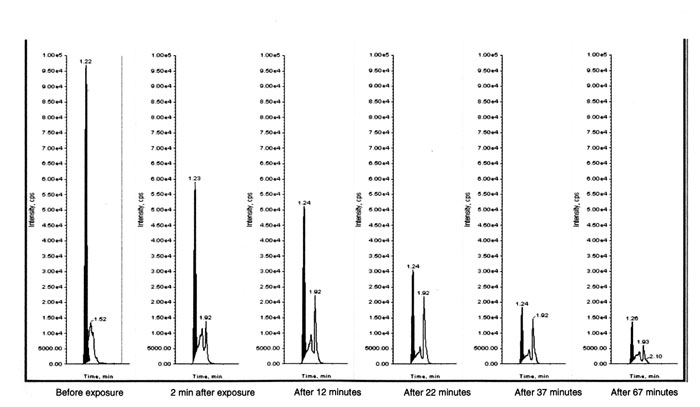
The chromatogram thus obtained is depicted in Fig.1.
 |
|
Fig.1 LC-MC/MS graph showing the bilirubin and photoisomer
peaks before and at different time points after exposure to spot LED
phototherapy light.
|
Comparative evaluation of phototherapy devices using
bilirubin: Similar to the method used for the confirmation of the HPLC
separation of bilirubin from photoconverted products, 1.2 mL vials
contain-ing 1 mL of methanolic solutions of bilirubin at the concentration
of 1µg/mL (serially diluted from the stock solution) were placed under all
phototherapy devices at the point of maximum irradiance at a distance of
35 cm and 10 µL of the bilirubin solution was aspirated from all the vials
before and after 15, 30, 45, 60, 90 and 120 min of exposure to light. All
the light sources were switched on at least 1 hour prior to the
experiment. The same bilirubin solution kept in the dark served as a
control during the experiment.
Data entry was done using Excel 2007 (Microsoft,
Redmond, WA, USA). Analysis was done by using Excel 2007 and SPSS 15.0
version for Windows. Data were presented as mean (SD) or number (%) as
appropriate. Friedman non-parametric two-way ANOVA was used to compare the
percentage reduction of bilirubin noted over a time period with the four
phototherapy devices. A P value of <0.05 was considered as
statistically significant.
Results
The device characteristics of the four phototherapy
devices tested are summarized in Table I. The spot
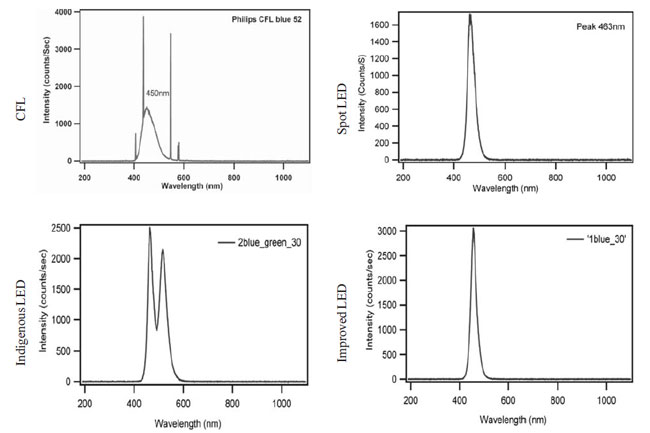
LED and improvised LED devices had similar range of emission spectra but
the peak emission spectra was slightly different (spot LED, 463nm;
improvised LED, 456nm). The indigenous LED device showed double peak
(464nm and 517nm) in the emission spectra due to the presence of blue and
green lamps. The spectral data for special blue CFL bulbs (Philips
Electronics India Pvt Ltd, India) showed a peak emission
spectrum of 450 nm with a slightly wider spectral range than the spot and
improvised LED devices (Fig. 2).
Table I Comparison of Different Phototherapy Devices
|
Phototherapy |
Emission spectral data (nm) |
Maximum |
Mean |
Decay of |
Area of |
|
device |
Peak |
Band |
Spectral |
irradiance |
irradiance# |
irradiance |
foot print of |
| |
emission |
width* |
range |
(µW/cm2/nm) |
(µW/cm2/nm) |
(µW/cm2/nm/day) |
light (cm2) |
| |
spectra |
|
|
(Mean ± SD) |
(Mean ± SD) |
|
|
|
Spot LED |
463 |
35 |
420-520 |
56.5 ± 1.9 |
26.8 ± 1.3 |
0.34 |
755 |
|
Indigenous LED |
464,*517 |
70 |
430-586 |
16.8 ± 0.1 |
10.8 ± 0.3 |
0.14 |
1800 |
|
Improvised LED |
456 |
28 |
420-520 |
39.7 ± 0.6 |
11.5 ± 0.3 |
0.04 |
2200 |
|
CFL |
450 |
60 |
400-550 |
37.5 ± 0.3 |
26 ± 0.1 |
0.32 |
1800 |
|
* Band width:
Absolute difference between the wavelengths at which the spectral
radiant intensity is 50 percent of the maximum power; # Mean
irradiance: Average of irradiance of the light falling within the
silhouette of the neonate. |
 |
|
Fig. 2 Emission spectra of different lamps.
|
Maximum irradiance: The average maximum
irradiance at the center and at four different perpendicular points at the
periphery of the different devices is shown in Table II. The
spot LED and the indigenous LED devices had the highest and lowest maximum
irradiances, respectively (56.5 and 16.8 µW/cm 2/nm).
Improvised LED and CFL units had almost equal irradiance at the center. In
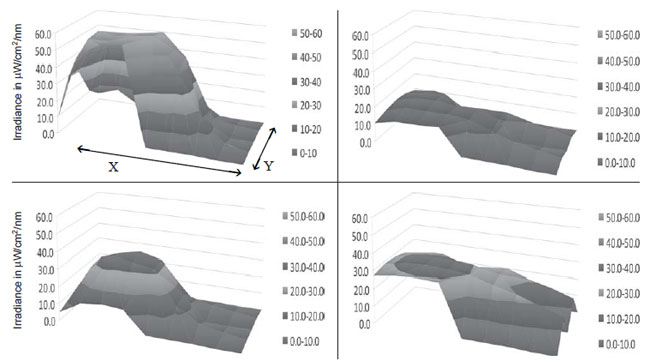
contrast to the high maximum irradiance observed at the center, the mean
irradiance (measured in those grids that fall within the silhouette of the
term baby) of the spot and improvised LED devices was low (Table
II). This unevenness in the distribution of irradiance is depicted
graphically in the surface irradiance plots. The CFL device had more
uniform distribution of irradiance (Fig. 3). The
decay of irradiance over a period of one month was highest in the spot LED
system (Table II). Improvised LED system had the least decay
of irradiance over time.
Table II
Comparison of Spectral Irradiance of Different Phototherapy Devices at the Center and at Four
Peripheral Perpendicular Points (µw/cm2/nm) (Mean ± SD)
| |
East |
West |
Center |
North |
South |
| Spot LED |
16.8 ± 0.9 |
22.3 ± 1.2 |
56.5 ± 1.9 |
0.8 ± 0.1 |
0.9 ± 0.1 |
|
Indigenous LED |
9.8 ± 0.1 |
10.3 ± 0.2 |
16.8 ± 0.1 |
3.5 ± 0.1 |
2.3 ± 0.0 |
|
Improvised LED |
6.1 ± 0.2 |
3.8 ± 0.2 |
39.7 ± 0.6 |
0.4 ± 0.1 |
1.1 ± 0.1 |
| CFL |
25.9 ± 1.1 |
27.9 ± 2.5 |
37.5 ± 0.3 |
19.5 ± 1.1 |
16.7 ± 0.9 |
East, West, North, South – four peripheral perpendicular points at a distance of 15cm breadth-wise (i.e. East/West)
and 30cm lengthwise (i.e. North/South) from the center.
|
 |
|
Fig.3 Surface irradiance plots for different phototheray
devices. (a) Spot LED, (b) Indigenous LED, (c) Improvised LED, (d)
CFL; X=60 cm, Y=30cm (size of the spacer board used)
|
 |
|
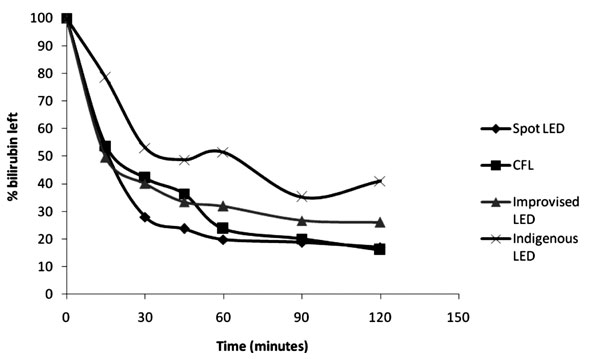
Fig. 4. Percentage of bilirubin left
over (in vitro) after exposure to light with different devices.
|
The 2D surface area of the term baby silhouette was 780
cm2. The surface area of the
foot print of the spot LED (diameter=31cm), indigenous LED, improvised LED
and CFL devices were 755, 1800, 1800, and 2200 cm2,
respectively. While the foot prints of the CFL, indigenous and improvised
LED lights covered the 2D silhouette of the term baby completely
(treatable surface area of 100%), the foot print of the spot LED light
covered only 55% of the term baby silhouette.
There was a significant difference in the amount of
bilirubin left after exposure to light over a 2-hour time period (%
reduction of bilirubin) among the four devices (P=0.001). At 15
minutes after exposure, only 50% of native bilirubin was left in the
sample, the amount was comparable for all the devices except for the
indigenous LED unit (Fig. 2). At 120 minutes, the
amount of bilirubin left was lowest for the CFL (16%) and spot LED (17%)
devices and highest for the indigenous LED unit (41%). The rate of
photoconversion reached a plateau after 60 min of light exposure with all
the four devices. The percent reduction of bilirubin observed with CFL
device after 60min of exposure was higher than that with improvised LED
despite a higher maximum irradiance of the latter (Fig. 2).
Discussion
The phototherapy devices differed in their physical and
photobiological properties. None of the devices tested showed harmful
spectrum in UV or IR range. The emission spectral ranges of all the blue
LED bulbs were narrow with peak emission spectrum very near to the peak
absorption spectrum of bilirubin. This characteristic has been well
emphasized previously by Vreman, et al. [7].
The irradiance at the center of the spot LED and the
improvised LED devices were high, the significance of which is not clear
given that bilirubin photoconversion could stagnate after certain level of
irradiance [8]. The existence of such saturation point is, however, still
debated. The uneven distribution of irradiance across the area of exposure
led to a drop in the mean irradiance to almost 50% and 25% of the peak
irradiance in spot and improvised LED, respectively. In addition, the foot
print of spot LED covered only 55% of the two-dimensional body surface
area. This has the potential to reduce the overall efficacy of the
phototherapy device. The concentration of irradiance centering on a
restricted area of foot print of light makes it necessary for the
healthcare provider to ensure that the baby and device are in proper
alignment. The other two devices, CFL and indigenous LED devices, had
wider distribution of irradiance across the foot print of light.
The decay of irradiance and thus the life span of any
bulb will depend on the amount of usage and on factors like operating
voltage, manufacturing defects, exposure to voltage spikes, frequency of
cycling on and off and ambient operating temperature. The phototherapy
bulbs showed decline in irradiance over a period of time more so for the
spot LED and CFL lamps. We presume higher consumption of amperage and
ineffective cooling of bulbs in CFL, and ineffective cooling in spot LED
devices when compared to other LED devices could have contributed to this
finding.
In vitro formation of lumirubin as a surrogate
marker for determining the efficacy of the device was studied previously
and neoBLUE LED was demonstrated to be superior [9]. Ennever, et al.
[10] showed much earlier that the tungsten halogen lamps and special blue
lamps generated higher lumirubin levels in comparison to those with broad
spectrum like the day light lamps. In our study, though the predominant
photoconversion product was estimated (formation of which was linear in
the initial part but subsequently had a plateau), we could not specify as
to whether the estimated product was lumirubin (structural isomer) or a
configurational isomer (both have same molecular weights).
All the devices displayed a linear and similar fall in
the bilirubin levels in the initial phase of the study except the
indigenous device which demonstrated linear but a slightly delayed fall.
In general, the percent reduction of bilirubin was more for the devices
with higher maximum irradiance save for the CFL unit which resulted in a
higher rate of bilirubin degradation than the improvised LED despite
having a slightly low maximum irradiance. This observation is intriguing
and is indeed difficult to explain as these data are based on exposure of
single sample. The experiment needs to be replicated to generate a robust
conclusion and extrapolation of these data to in vivo environment
is difficult as it is a more dynamic environment with continuous formation
of bilirubin and excretion of photoproducts.
This study is the first of its kind in India. There are
no studies from our country which have looked at almost all the parameters
that affect the efficacy of the phototherapy devices in an in vitro
scenario. The method of estimation of bilirubin and its photo-products
using LC MS/MS technology is a novel high precision technique. This method
used a new technique "hydrophilic interaction chromatography" (HILIC) to
resolve hydrophobic bilirubin from its isomers having similar molecular
weights.
The limitations of the study were use of fixed band
irradiance meter with its attendant limitations but as it estimates
irradiance within the therapeutic wavelength range, this should be the
appropriate device [11,12]. The method used for mapping irradiance across
the foot print of light may not have been perfect but conformed to
standards laid by Vreman, et al. [1], but still matched what would
be relevant for clinical practice.
In conclusion, the available phototherapy devices
differed considerably. Combination of characteristics as enlisted in this
study should be considered in toto before judging the efficacy of
the unit. An ideal device should have a maximum and mean irradiance of
>30µW/cm2/nm with the foot
print of the light covering an area of at least 60×30cm and distribution
of irradiance being uniform across the foot print of light, have least
decay of irradiance, and have high rate of bilirubin degradation. CFL had
many if not all the characteristics in this in vitro study.
Knowledge about in vivo performance of these phototherapy devices
and estimation of photoisomers would further help in characterizing the
efficacy of different phototherapy devices. There is a need for regulatory
bodies to define standard guidelines to ensure that only efficacious
phototherapy devices are marketed.
Acknowledgments: Dr AK Ravi and Ph D students of
the Department of Ocular Pharmacology for their valuable help in analyzing
photo-degradation products.
Contributors: AKD conceived and designed the study
and revised the manuscript for important intellectual content. He will act
as guarantor of the study. SS conducted the experiments, analyzed the data
and drafted the paper. MJS, RA and VKP provided inputs regarding the
design and revised the manuscript for intellectual content. Emission
spectral data was recorded by PK and was supervised by GVP. TV and his
team designed the methodology to isolate and quantify the bilirubin and
photoisomers using LC-MS/MS. The final manuscript was approved by all
authors.
Funding: None.
Competing interests: The phototherapy devices were
supplied free of cost by Phoenix Medical systems Pvt Ltd, Chennai, India (CFL
and LED Spot phototherapy unit); SriChakra Scientifics, Hyderabad. India
(Indigenous LED phototherapy "Photolux"); and Fanem Inc, Brazil
(Improvised LED Phototherapy ). None of the manufacturers had any role in
study design, collection of data, analysis, and interpretation of results.
|
What is Already Known?
• Phototherapy devices differ in the maximum
irradiance.
What This Study Adds?
• Phototherapy devices also differ in other key physical and
photobiological characteristics that influence the efficacy of the
device.
|
References
1. Vreman HJ, Wong RJ, Murdock JR, Stevenson DK.
Standardized bench method for evaluating the efficacy of phototherapy
devices. Acta Pediatrica.2008;97:308-16.
2. Dicken P, Grant LJ, Jones S. An evaluation of the
characteristics and performance of neonatal phototherapy equipment.
Physiol Meas. 2000;21:493-503.
3. Hart G, Cameron R. The importance of irradiance and
area in neonatal phototherapy. Arch Dis Child Fetal Neonatal Ed.
2005;90:F437-40.
4. BARTrial Study Group. Variability of Irradiance
Levels of Phototherapy in Jaundiced Preterm Infant. PAS 2009. Available
from http://www.abstracts2view.com/pas/Accessed September 23, 2009.
5. Pejaver RK, Vishwanath J. An audit of phototherapy
devices. Indian J Pediatr. 2000;67:883-4.
6. International Electrotechnical Commission. Medical
Electrical Equipment - Part 2-50: Particular Requirements for the Safety
of Infant Phototherapy Equipment. 2000. IEC 60601-2-50.
7. Vreman HJ, Wong RJ, Stevenson DK. Light-emitting
diodes: a novel light source for phototherapy. Pediatr Res. 1998; 44:
804-9.
8. Tan KL. The pattern of bilirubin response to
phototherapy for neonatal hyperbilirubinemia. Pediatr Res.1982;16:
670-4.
9. Okada H, Abe T, Etoh Y, Yoshino S, Kato I, Iwaki T,
et al. In vitro production of bilirubin photoisomers by light
irradiation using neoBLUE. Pediatr Int. 2007;49:318-21.
10. Ennever JF, Sobel M, McDonagh AF, Speck WT.
Phototherapy for neonatal jaundice: In vitro comparison of light sources.
Pediatr Res. 1984;18:667-9.
11. Maisels MJ. Why use homeopathic doses of
phototherapy? Pediatrics. 1996;98:283-7.
12. Costarino AT, Ennevar JF, Baumgart S, et al.
Effect of spectral distribution on isomerisation of bilirubin in-vivo. J
Pediatr. 1985;17:125-8.
|
|
|
 |
|

