|
|
|
Indian Pediatr 2014;51:
780-784 |
 |
Zinc Supplementation for Prevention of Acute
Respiratory Infections in Infants:
A Randomized Controlled Trial
|
|
Akash Malik, Davendra K Taneja,
#Nivedhita Devasenapathy
and *K Rajeshwari
From Departments of Community Medicine and
*Pediatrics, Maulana Azad Medical College, New Delhi, India; and #Indian
Institute of Public Health, Gurgaon, Haryana, India.
Correspondence to: Dr Akash Malik, National RMNCH+A
Unit, Room No. 107 D, Ministry of Health and Family Welfare, Government
of India, Nirman Bhawan, New Delhi 110 001, India.
Email:
[email protected]
Received: May 27, 2014;
Initial review: June 13, 2014;
Accepted: August 12, 2014.
Trial Registration No. CTRI/2010/091/001417
|
Objective: To study the effect of 2 weeks of prophylactic zinc
supplementation on incidence and duration of acute respiratory
infections.
Design: Randomized double blind controlled trial.
Setting: Community based; urban resettlement area
in North-East Delhi, India.
Participants: 272 children aged 6-11 months with
acute respiratory infections. Children receiving zinc supplement within
the past 3 months, severely malnourished, immuno-deficient, on steroid
therapy, with severe illness requiring hospitalization, or children of
families likely to migrate from the study area were excluded.
Intervention: Placebo (syrup base) or zinc (20
mg/5 mL elemental zinc as zinc sulfate) orally given for a period of 2
weeks.
Main outcome measure(s): Incidence, type and
duration of acute respiratory infections, and adverse effects.
Results: No effect on incidence of acute
respiratory infections was noted. A decrease of 15% (0.78-0.94) in days
and 12% (0.78-0.94) in duration of episode in acute respiratory
infections was observed. Incidence of acute lower respiratory infections
decreased by 62% (0.26-0.36) and the effect remained for full five
months of follow up. There were no drop outs due to side effects.
Conclusions: Prophylactic zinc supplementation
for two weeks may reduce the morbidity due to acute lower respiratory
infections but not overall rate of acute respiratory infections in
infants aged 6-11 months in similar populations.
Keywords: Micronutrient, Pneumonia, Public
health, Prophylaxis.
|
|
Zinc is a vital micronutrient in humans and is
essential for protein synthesis, cell growth, and differentiation and
thus is important for functioning of the immune system [1]. Mild to
moderate zinc deficiency is common in several developing countries,
including India, because the commonly consumed staple foods have low
zinc contents and are rich in phytates, which inhibit the absorption and
utilization of zinc [2]. Strong evidence for a causal relationship
between zinc deficiency and childhood infections has come from
randomized controlled trials of zinc supplementation [3].
Acute respiratory infections (ARIs) especially Acute
lower respiratory infections (ALRIs), are among the leading causes of
death in children under the age of 5 years [4-6]. Zinc deficiency is
projected to be responsible for 118,000 thousand deaths in children less
than 5 years in developing countries [7]. Recent trials and
meta-analyses have demonstrated that zinc supplementation both
therapeutic and prophylactic reduces the duration, severity and
incidence of ARIs [8,9].
However, most of these trials have used continuous supplementation, in a
wide age group and produced variable results. The current study aimed to
evaluate whether zinc prophylaxis for a short duration has any role in
reducing the morbidity due to ARIs in apparently healthy infants of 6-11
months of age.
Methods
This was a community-based, randomized, double-blind,
parallel-arm placebo-controlled trial, conducted from 1st January 2011
to 15th January 2012. We included all infants 6-11 months of age
residing in Gokulpuri, an urban resettlement colony in North East
District of Delhi, India, who were likely to stay till the completion of
the study. Gokulpuri has a predominantly migrant population of about
23000, the majority belonging to the middle and lower socioeconomic
strata. To achieve the final sample size, additional children were
recruited from adjacent area of Gangavihar which has a similar
population.
The study was approved by the Institutional Ethical
Committee of Maulana Azad Medical College and Associated Hospitals, New
Delhi.
We hypothesized that zinc supplementation for 2 weeks
will reduce the incidence of ARIs in subsequent months. We excluded
childred receiving zinc supplement in the past 3 months, those who were
severely malnourished, known immuno-deficient or on steroid therapy,
severely ill children requiring hospitalization, and children of
families likely to migrate from the study area. A house-to-house survey
was done at the beginning of the study to identify and recruit the
eligible infants. The study purpose was explained and an informed
consent was obtained from parents of all infants before they were
recruited. The recruitment was done during first two weeks of January
and July followed by subsequent five months of follow-up.
Intervention: The liquid
preparations were prepared by Abyss Pharma, Delhi. Each 5 mL of the
preparation contained placebo (syrup base) or zinc (20 mg elemental zinc
as zinc sulfate). The syrups were of similar color (orange), taste
(orange flavored), and consistency, and were packaged in similar
bottles. We randomized the treatment allocation by simple randomization
using computer generated random numbers (Excel 2010). The bottles were
labeled with serial numbers after randomization in the Department of
Community Medicine, MAMC, without the knowledge of the field
investigator. The field investigator and parents were blinded to the
treatment allocation till the end of follow-up period. The mothers
received the bottles with labeled serial numbers and names. The field
investigator administered the first dose of the intervention at the time
of recruitment and advised the mother to give 5 mL of syrup (using
standard 5 mL plastic spoon) daily to the infant for the remaining 13
days. Subsequently visits were made on the 7th and the 14th day to
ensure compliance. In case the syrup had not been given regularly, a
maximum of one week was given to complete the dosages. We collected data
for any possible side effects as reported by the caregivers during these
visits. To ensure that the child did not receive additional doses of
zinc, we provided mothers with identity cards indicating the study title
and that the infant was participating in the study. These cards were to
be produced whenever the child was taken to any medical practitioner.
Outcomes and Follow up: The primary outcome was
the incidence of ARIs per child-year. Secondary outcomes included
incidence of Acute Upper Respiratory Infections (AURI) and ALRI
per child-year, duration of ARIs, and side effects. AURI was
diagnosed if the child had cough or cold with or without fever. ALRI was
diagnosed if the child had symptoms of cough with difficult and/or rapid
breathing or chest indrawing as informed by the caregiver [10].
Duration was assessed as the number of days with
ARIs and as mean number of days an ARI episode lasted. A baseline
assessment was done at the time of recruitment which included weight and
length measurements using a Salter weighing Scale (up to 100 g) and an
infantometer (up to 1 mm), respectively. All the outcomes were assessed
by a trained field investigator based on history by caregiver.
Follow-up for ARIs began at the 15th day post-
intervention. Each child was followed up fortnightly (± 3) days and the
follow-up continued till 5 months after completion of zinc/placebo
supplementation. At each follow-up, mother/caregiver was asked
about history of ARIs during the previous 15 days. Recovery from an ARI
episode was considered when the last day of ARIs was followed by a
72-hour ARI-free period [10]. Subsequent episodes were considered to be
new ARIs episodes.
Sample Size: For sample size calculation,
incidence of ARI was taken as 5.5 episodes (SD = 3.15) per child-year as
per previous studies [11].
Thus, for a 20% reduction in the incidence of ARI (a
0.05 and power 80%), we required 258 infants (129 in each group). Taking
into account possible 5% attrition, the final sample size was 272.
Statistical Analysis: The data were
collected and checked for accuracy on a daily basis and entered in SPSS
version 16. The incidence was expressed as episodes per child per year.
The counts were expressed by means and standard deviation. Difference
between means was tested using t-test, for normally distributed data or
Mann Whitney U test, for skewed data.
Generalized Estimating Equations (GEE) were used to
obtain an incident rate ratio with 95% confidence intervals, in order to
compare month-wise number of episodes and duration of ARIs using Poisson
log linear distribution, by intention to treat analysis. The
exchangeable working correlation matrix was selected for all the
outcomes. We included all children who had taken at least two doses of
the intervention for the analyses. The follow-up visits for which the
infant outcomes were not available were imputed using the worst case (2
episodes of ARI) and best case scenarios (no episodes). As it did not
change the study results, the missing data were excluded from the final
analysis. We decided to adjust the incident rate ratio for covariates
which appeared to be different at baseline in the two groups. We also
decided to compare the month-wise mean episodes of ARIs in the two
groups.
Socioeconomic status was assessed using the Modified
Kuppuswamy Scale (based on education and occupation of family head and
total family income) modified for Consumer Price Index for industrial
workers of India for 2011 [12].
Z-scores for length and weight were calculated using WHO
reference tables for length and weight [13,14].
Results
From a total of 3155 households identified during the
house to house survey, we assessed 272 infants for eligibility and all
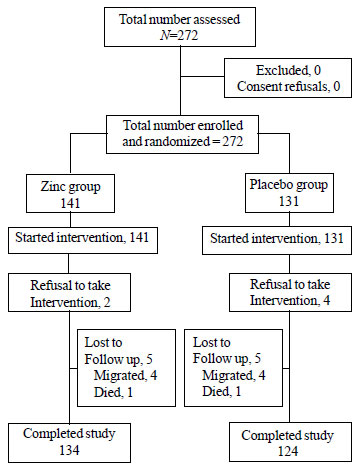
were recruited (Fig. 1). Infants in both the groups shared
similar baseline characteristics (Table I). Seven families
which migrated during the study period also shared similar baseline
characteristics. Final analyses included 134 infants in the zinc group
and 124 in the placebo group, who had completed the study. The mean
number of follow-ups was 10 in each group. A total of 19 infants (13.5%)
in zinc group and 26 infants (20%) in placebo group were given
additional one week to complete the intervention as they were found to
be initially non-compliant. The parents of infants who refused to be the
part of the study even after this period were excluded from the analysis
as refusals.
TABLE I Baseline Characteristics of the Study Participants
|
Characteristic |
Zinc |
Placebo |
|
(n=141) |
(n=131) |
|
Male gender, n (%) |
67 (47.5%) |
68 (51.9%) |
|
Mean age, mo; mean (SD) |
8.77 (1.73) |
8.76 (1.86) |
|
Socioeconomic status |
|
Upper & upper middle |
43 (30.5%) |
34 (26%) |
|
Lower middle and lower |
98 (69.5%) |
97 (74.1%) |
|
Length for age, Z score* |
-1.76 (1.46) |
-1.69 (1.48) |
|
Stunted, n (%) |
51(36.0%) |
54 (41.0%) |
|
Weight-for-age Z score* |
-1.50 (1.15) |
-1.58 (1.21) |
|
Wasted; n (%) |
43(30.0%) |
53 (41.0%) |
|
*Mean (SD) |
 |
|
Fig. 1 Trial flow.
|
Out of the total 862 episodes observed, 424 episodes
occurred in the zinc group and 438 in the placebo group, accounting for
an incidence of 7.84 and 8.70 per child- year, respectively, at the end
of 5 months (Table II). GEE regression model showed that
there was a non-significant reduction of 9% (Adjusted IRR 0.91, 95% CI
0.81-1.02) in episodes of ARIs in the zinc group as compared to the
placebo group.
TABLE II Effect of Zinc Supplementation on Incidence and Duration of ARIs in the Study Participants
|
Outcome |
Zinc |
Placebo |
Adjusted
IRR* |
|
Child years observed |
54.8 |
50.2 |
|
|
ARI Incidence$ |
7.8 |
8.7 |
0.9 (0.81-1.02) |
|
AURI Incidence$ |
7.2 |
7.2 |
1.0 (0.89-1.14) |
|
ALRI Incidence$ |
0.5 |
1.5 |
0.3 (0.26-0.56) |
|
Days# with ARI |
11.4 (6.6) |
14.7 (8.0) |
0.8 (0.78-0.94) |
|
Days# per episode ARI |
3.6 (1.6) |
3.8 (1.2) |
0.8 (0.78-0.94) |
|
*Incident rate ratio adjusted for wasting (95% CI); #Mean
(SD); $episode/child/yr. |
When types of ARIs were analyzed separately (Table
II), we found a non-significant increase of 1% in the episodes of
AURIs (Adjusted IRR 1.01, 95% CI: 0.89-1.14). However a significant
decrease of 62% in the episodes of ALRIs (Adjusted IRR 0.38, 95% CI:
0.26-0.36) was observed in the zinc group.
Zinc supplementation led to a significant reduction
of 15% (Adjusted RR 0.85, 95% CI: 0.78-0.94) in days with ARIs. There
was also a significant reduction of 12% in duration per episode of ARIs
(Adjusted RR 0.88, 95% CI: 0.78-0.94) observed in the zinc group (Table
II). A subgroup analysis on wasted and stunted infants showed
similar effects of prophylactic zinc (Web Table I).
After the second month the episodes were almost
similar in the two groups. Zinc prophylaxis significantly reduced the
incidence of ALRI for all months of follow-up (Web Table II).
Reported side effects were diarrhea, vomiting and
constipation. The percentage of children reporting these were 9%, 10.4%
and 1.5%, respectively in the zinc group and 7.3%, 4.8% and 0%,
respectively in the placebo group; the difference was non-significant. A
death due to diarrhea was reported in the zinc group three months after
recruitment. Verbal autopsy revealed severe dehydration due to
non-administration of oral rehydration solution or home available
fluids.
Discussion
We observed a significant reduction in duration of
all forms of ARIs after 14 days of zinc supplementation (20 mg/day). A
significant decrease of 62% in the episodes of ALRIs was observed.
The major limitation of this study is that serum zinc
levels were not done to assess the deficiency and the subsequent effect
on serum zinc levels. Nevertheless, previous studies in similar
populations of Delhi have shown that zinc deficiency is prevalent to the
extent of 73.3% for values less than 10.4 µmol/L and 33.8% for values
less than 9 µmol/L [15]. Moreover, in our study the proportion of
stunted infants was more than 20%, which suggests an elevated risk of
zinc deficiency since stunting is a proxy indicator of zinc deficiency
in population studies [16]. Thus the results of this study may be
extrapolated to similar zinc deficient populations only.
Previous studies done in healthy infants also
observed a non-significant or no reduction in the total number of
episodes of respiratory illness in the zinc group [17-21].
A meta-analysis [22] showed that, the children
receiving zinc had fewer attacks of ARI (RR-0.92, 95% CI: 0.85-0.99),
and fewer days with all ARI (RR-0.95, 95% CI: 0.84-1.07). However, the
authors excluded studies with short-course prophylaxis in this
meta-analysis.
Osendarp, et al. [19] reported 70% fewer
episodes of ALRIs with zinc prophylaxis in healthy infants with low
serum zinc levels at baseline. Though serum zinc levels were not
measured in the current trial, the study population is expected to have
low serum zinc levels [15,23]. However, in the above study, continuous
zinc prophylaxis was given, ranging from 5 to 12 months which is in
contrast to current trial. With the similar study setting, sample size
and short course zinc prophylaxis of zinc (20 mg/d for 2 weeks), Rahman,
et al. [10] reported that the incidence and prevalence of ALRI
were significantly higher in the zinc group than in the placebo group
after 6 months of follow-up. Other trials which had given zinc
prophylxis for continuously long durations reported either a
non-significant or no reduction in incidence of ALRI [24-26].
The meta-analyses of continuous and short course
zinc proplylaxis on the other hand have concluded that zinc prophylaxis
significantly reduces the incidence of ALRI [8,9,26-28]. Bhutta, et
al. [9] also showed that point estimates of effects were not
different in continuous and short-course zinc prophylaxis trials [9].
Despite different duration of zinc prophylaxis used in the above
studies, the effect on ALRI was either similar or better in the current
trial.
This trial on short course prophylactic zinc
supplementation for 2 weeks in infants of 6-11 months has shown to cause
a large decrease the incidence and duration of ALRIs in subsequent 5
months. Zinc prophylaxis in zinc deficient populations may significantly
decrease morbidity due to ALRIs. The results of this study have
important cost- and operational- implications as short-course
prophylaxis of zinc in adequate dose might be more feasible than
continuous therapy.
Contributors: AM: formulated the research
question and contributed to study design, carried out the field
investigations and initial data analyses, and wrote the initial draft;
DKT: contributed to study design, and methodology and revised the
manuscript; ND: supervised the data collection, analyzed the data, and
interpreted the findings; KR; supervised the clinical data collection
and management, and contributed to methodology. all authors approved the
final manuscript.
Funding: Indian Council of Medical
Research, Department of Health Research (Ministry of Health and Family
Welfare) Government of India. Ref No. 3/2/2011/PG-thesis-MPD-10.
Competing interests: None stated.
|
What is Already Known?
• Zinc supplementation for >3 months reduces
the incidence and severity of acute lower respiratory tract
infection but not overall acute respiratory tract infection in
children aged 1-5 years.
What This Study Adds?
• Short-course (2 weeks) prophylactic zinc
supplementation reduced acute lower respiratory tract infection
morbidity in apparently healthy infants of 6 to 11 months over 5
months of follow-up.
|
References
1. Biesel WR. Single nutrient and immunity. Am J Clin
Nutr. 1982;35:417-68.
2. Black RE. Zinc deficiency, immune function, and
morbidity and mortality from infectious disease among children in
developing countries. Food Nutr Bull. 2001;22: 155-62.
3. Baqui AH, Black RE, Walker CLF, Shams A, Yunus M.
Effect of zinc supplementation started during diarrhea on morbidity and
mortality in Bangladeshi children: community randomized trial. BMJ.
2002;325:1059-65.
4. Pneumonia, the forgotten killer of children
Geneva: UNICEF/ WHO; 2006. Available from:
http://whqlibdoc.who.int/publications/2006/9280640489_eng. pdf.
Accessed April 15, 2014.
5. Brian G. Global action plan for prevention and
control of pneumonia (GAPP). Bull World Health Organ. 2008;86:322.
6. Walker CF, Rudan I, Liu L, Nair H, Theodoratou E,
Bhutta ZA, et al. Global burden of childhood pneumonia and
diarrhoea. Lancet. 2013;381:1405-16.
7. Roth DE, Caulfield LE, Ezzati M, Balck RE. Acute
lower respiratory infections in childhood: Opportunities for reducing
the global burden through nutritional interventions. Bull World Health
Organ. 2008;86:356-64.
8. Brown KH, Sonja YS, Peerson JM, Baker SK. Does
preventive zinc supplementation of infants and young children affect
their risk of selected illnesses, survival, and physical growth. Food
Nutr Bull. 2009;30:S12-S40.
9. Zinc Investigators’ Collaborative Group: Bhutta
ZA, Black RE, Brown KH, Gardner JM, Gore S, et al. Prevention of
diarrhea and pneumonia by zinc supplementation in children in developing
countries: Pooled analysis of randomized controlled trials. J Pediatr.
1999;135:689-97.
10. Rahman MM, Vermund SH, Wahed MA, Fuchs GJ, Baqui
AH, Alvarez JO. Simultaneous zinc and vitamin A supplementation in
Bangladeshi children: randomized double blind controlled trial. BMJ.
2001;323:314-8.
11. Zaman K, Baqui AH, Yunus M, Sack RB, Bateman OM,
Chowdhury HR, et al. Acute respiratory infections in children: A
community-based longitudinal study in rural Bangladesh. J Trop Pediatr.
1997;43:133-7.
12. Kuppuswamy B. Manual of Socioeconomic Status
(Urban), Manasayan, Delhi, 1981.
13. World Health Organization. Child growth
standards. Weight-for-age. Available from:
www.who.int/childgrowth/standards/weight_for_age/en/index.html.
Accessed April 14, 2014.
14. World Health Organization. Child growth
standards. Height-for-age. Available at:
http://www.who.int/childgrowth/standards/height_for_age/en/index.html.
Accessed April 14, 2014.
15. Dhingra U, Hiremath G, Menon VP, Dhingra P,
Sarkar A, Sazawal S. Zinc Deficiency: Descriptive epidemiology and
morbidity among preschool children in peri-urban population in Delhi,
India. J Health Popul Nutr. 2009;27:632-9.
16. de Benoist B, Darnton-Hill I, Davidsson L,
Fontaine O, Hotz C. Conclusions of the joint WHO/UNICEF/IAEA/IZiNCG
interagency meeting on zinc status indicators. Food Nutr Bull.
2007;28:S480-4.
17. Rosado JL, Lopez P, Mufioz E, Martinez H, Allen
LH. Zinc supplementation reduced morbidity, but neither zinc nor iron
supplementation affected growth or body composition of Mexican
pre-schoolers. Am J Clin Nutr. 1997;65:13-9.
18. Ruel MT, Rivera JA, Santizo MC, Lonnerdal B,
Brown KH. Impact of zinc supplementation on morbidity from diarrhea and
respiratory infections among rural Guatemalan children. Pediatrics.
1997;99:808-13.
19. Osendarp JM, Santosham M, Black RE, Wahed MA, van
Raaij JMA, Fuchs GJ. Effect of zinc supplementation between 1 and 6 mo
of life on growth and morbidity of Bangladeshi infants in urban slums.
Am J Clin Nutr. 2002;76:1401-8.
20. Long KZ, Montoya Y, Hertzmark E, Santos JI,
Rosado JL. A double-blind, randomized, clinical trial of the effect of
vitamin A and zinc supplementation on diarrheal disease and respiratory
infections in children in Mexico City, Mexico. Am J Clin Nutr.
2006;83:693-700.
21. Heinig MJ, Brown KH, Lönnerdal B, Dewey KG. Zinc
supplementation does not affect growth, morbidity, or motor development
of US term breastfed infants at 4-10 mo of age. Am J Clin Nutr.
2006;84:594-601.
22. Aggarwal R, Sentz J, Miller MA. Role of zinc
administration in prevention of childhood diarrhoea and respiratory
illnesses: A meta-analysis. Paediatrics 2007; 119:1120-30.
23. Bhandari N, Behl R, Taneja S, Strand T, Molbak K.
Effect of routine zinc supplementation on pneumonia in children aged 6
months to 3 years: randomised controlled trial in an urban slum. BMJ.
2002;324:1358-63.
24. Baqui AH, Zaman K, Persson LA, Arifeen SE, Yunus
M, Begum N, et al. Simultaneous weekly supplementation of iron
and zinc is associated with lower morbidity due to diarrhoea and acute
lower respiratory infection in Bangladeshi infants. J Nutr.
2003;133:4150-7.
25. Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J,
LeClerq SC, Adhikari R, et al. Effect of daily zinc
supplementation on child mortality in southern Nepal: a community-based,
cluster randomised, placebo controlled trial. Lancet. 2007;370:1230-9.
26. Roth DE, Richard SA, Black RE. Zinc
supplementation for the prevention of acute lower respiratory infection
in children in developing countries: meta-analysis and meta-regression
of randomized trials. Int J Epidemiol. 2010;39:795-808.
27. Yakoob MY, Theodoratou E, Jabeen A, Imdad A,
Eisele TP. Preventive zinc supplementation in developing countries:
impact on mortality and morbidity due to diarrhoea, pneumonia and
malaria. BMC Public Health 2011;11:S23.
28. Mathew JL, Patwari AK, Gupta P, Shah D, Gera T,
Gogia S, et al. Acute respiratory infection and pneumonia in
India: a systematic review of literature for advocacy and action:
UNICEF-PHFI series on newborn and child health, India. Indian Pediatr.
2011;48:191-218.
|
|
|
 |
|

