|
|
|
Indian Pediatr 2013;50:
934-938 |
 |
Effectiveness and Safety of Intravenous
Iloprost for Severe Persistent Pulmonary Hypertension of the
Newborn
|
|
Waricha Janjindamai, Anucha Thatrimontrichai, Gunlawadee Maneenil,
Prasin Chanvitan and
Supaporn Dissaneevate
From the Division of Neonatology, Department of Pediatrics, Faculty
of Medicine,
Prince of Songkla University, Hat Yai, Songkhla, Thailand.
Correspondence to: Dr Waricha Janjindamai, Division of Neonatology,
Department of Pediatrics, Faculty of Medicine,
Prince of Songkla University, 15 Kanjanavanit Road, Hat Yai, Songkhla,
Thailand, 90110.
Email:
[email protected]
Received: September 14, 2012;
Initial review: October 11, 2012;
Accepted: March 30, 2013.
|
Objective: The aims of this study were to determine the
effectiveness (oxygenation), safety (hemodynamic status) and short term
outcomes of intravenous iloprost (IVI) administration as a rescue
therapy in severe persistent pulmonary hypertension of the newborn
(PPHN).
Design: Retrospective medical records review.
Setting: Tertiary neonatal intensive care unit at
Songklanagarind Hospital, Songkhla Province, Hat Yai, Thailand.
Participants: Newborns who received IVI as an
adjunctive therapy for treatment of severe PPHN, as defined by an oxygen
index (OI) of >20 and without response to conventional therapies.
Main Outcome Measures: The change of OI and
alveolar-arterial oxygen difference before and after commencement of
IVI.
Results: 33 neonates with severe PPHN at a median
gestation of 39 weeks and a baseline OI of 40 (range, 21-101) received
IVI. The median OI and alveolar-arterial oxygen difference had a
statistically significant decrease after 2 hours of treatment and
continued to decline thereafter (P<0.05). All infants received
one or more inotropic medications and volume expanders to provide blood
pressure support with no statistically significant difference of blood
pressure and heart rate before and after IVI treatment. The mortality
rate was 15.2%, all of them had initially severe hypoxemia with a median
OI of 53.6.
Conclusions: IVI may be effective in improving
oxygenation and should be considered as a rescue therapy for infants
with severe PPHN, especially in a limited resource environment with no
inhaled nitric oxide available. Systemic hypotension may be a cause for
concern.
Keywords: Newborn, Persistent fetal circulation syndrome,
Prostacyclin; Pulmonary Hypertension.
|
|
Persistent pulmonary hypertension of the newborn
(PPHN) is a life-threatening neonatal pathology with high mortality [1]
and high neurologic disability [2]. The primary goal of treatment for
PPHN is selective pulmonary vasodilation. Inhaled nitric oxide (iNO)
therapy is effective [3], but is not available in developing countries.
Alternative, less expensive treatments are being sought. Iloprost, a
stable analog of endogenous prostacyclin, has been used successfully in
its intravenous and aerosolized forms for treating adults and children
with pulmonary hypertension (PH). Aerosolized prostacyclin was found to
be a potent pulmonary vasodilator in patients with acute respiratory
failure, exerting preferential vasodilatation in well-ventilated lung
regions [4]. In patients with PH who deteriorated while being treated
with aerosolized iloprost substantial improvement in exercise capacity
was seen after switching to continuous intravenous iloprost (IVI) [5].
The inhaled route of administering iloprost has fewer side-effects than
the intravenous approach and obviates the need of a permanent central
venous access [6-9]. The objectives of this study were to determine the
oxygenation, hemodynamic status and short term outcome of IVI.
Methods
A retrospective medical records review was performed
in newborns who received IVI as an adjunctive therapy for treatment of
PPHN between December 2007 and December 2011 at the neonatal intensive
care unit of Songklanagarind Hospital which is the major tertiary care
institution in southern Thailand. Patient selection was based upon a
diagnosis of PPHN by echocardiography with evidence of a structurally
normal heart and suprasystolic pulmonary hypertension, with
right-to-left intracardiac shunting [10]. Exclusion criteria included
the presence of other anomalies (including congenital heart disease,
congenital diaphragmatic hernia, and lung hypoplasia syndromes) or
chromosomal anomalies and infants who were not diagnosed by
echocardiography. The study was approved by the Ethics Committee Board
of Prince of Songkla University and informed consent was obtained from
the parent or guardian before starting the IVI.
The primary outcome was the effect of IVI on
oxygenation [decreased oxygen index (OI) and alveolar-arterial oxygen
difference (AaDO 2)] and
hemodynamic status (blood pressure, heart rate and needed inotropic
medications and volume expanders) over a 72-hour period after
commencement of treatment. Secondary out-comes included the mortality
rate, duration of ventilatory support and bronchopulmonary dysplasia
(BPD).
All infants who were diagnosed as having PPHN were
treated with the conventional therapies including HFOV, inotropic
therapy such as dobutamine, dopamine, epinephrine or norepinephrine and
sedation, because of the non-availability of ECMO and iNO. Infants with
severe PPHN, as defined by an OI of >20 at the time of diagnosis, who
did not respond to the conventional therapies were considered for
administration of IVI (Ilomedin, Bayer Schering Pharma, Spain). The
starting dose for IVI was between 0.5 and 3.0 ng/kg/minute with
maintenance doses of 1-10 ng/kg/minute. The dosage was titrated
according to the clinical response and increased in increments of 0.5-1
ng/kg/minute. The IVI was prepared and diluted with isotonic saline
solution resulting in a 1 mcg/ml solution. It was administered through a
central vein using a pump system. The dosage was titrated up
sufficiently to achieve substantial clinical improvement while
maintaining adequate systemic blood pressure for infants responding to
treatment. These infants were defined as the responder group. If the
infants did not respond to IVI as indicated by no improvement in OI or
PaO 2 after commencement of
IVI within 12-24 hours they were defined as the non-responder group and
another pulmonary vasodilator was administered. IVI was discontinued if
clinical deterioration occurred, defined by refractory hypotension not
responding to volume expander or inotropic agents [11]. After oxygenation
improvement, indicated by an improvement in OI (OI<10), the IVI was
tapered off by 0.5-1 ng/kg/minute and finally discontinued. The
decisions to commence or adjust an alternative inotropic agent and to
wean assisted ventilation or supplemental oxygen treatment depended on
the discretion of the attending neonatologist. Concomitant drug therapy
consisted of sedatives, analgesics and antibiotics or other vasodilators
as required, adjusted to the individual needs of each infant.
The demographic data and information on various study
characteristics was collected from hospital records. The time of IVI
commencement, minimum and maximum dose, and duration of treatment were
also obtained. The definition of BPD was defined as a need for
supplemental oxygen for at least 28 days and times of point assessment
were at 36 weeks’ postmenstrual age for babies born before 32 weeks’
gestational age or at 56 days of life for babies born at or beyond 32
weeks’ gestational age or discharged home, whichever came first [12].
Cranial ultrasonography was performed at the first and fourth week by
the pediatrics radiologist and was classified into 4 grades of severity
[13]. Hearing screening tests were performed using the otoacoustic
emission technique (MADSEN AccuScreen, AURICAL, GN Otometrics, UK) at
the time of discharge.
Statistical analysis: The data and
clinical parameters were expressed as mean (SD) or median (range). The
Epicalc package in R Software version 2.13.1 was used for statistical
analysis. The Wilcoxon rank-sum test and Fisher’s exact test were used
to compare the difference between the responder and non-responder of
iloprost-treated severe PPHN. Since the data were not normally
distributed, the Wilcoxon signed-rank test was applied to compare before
and after IVI application with OI, SpO 2,
PaO2, AaDO2,
blood pressure and heart rate. A P<0.05 was considered
statistically significant.
Results
During the study period, 41 and 35 infants were
diagnosed as PPHN and severe PPHN, respectively, who needed the IVI
treatment. Thirty-three (33/35, 94.3%) infants were diagnosed as PPHN by
echocardiography and enrolled in the study. There were no dropout cases
during the study period due to severe hypotension not responding to
inotropic agents or volume expander. 16 (48.5%) were referred from other
hospitals. The most common etiology of the PPHN was meconium aspiration
syndrome (MAS) (n=18, 54.6%) (Table I).
TABLE I Baseline Characteristic of Study Subjects
|
Male/female, (n) |
22/11 |
|
Birthweight, g |
3,120 (1,600-4,310) |
|
Gestational age, wk, |
39 (30-44) |
|
Apgar score: 1 min; 5 min |
6 (1-10); 8 (1-10) |
|
Cesarean section, n (%) |
23 (69.7) |
|
Primary cause of PPHN, No. (%) |
|
|
Meconium aspiration syndrome |
18 (54.6) |
|
Pneumonia
|
12 (36.4) |
|
Sepsis |
2 (6) |
|
Idiopathic pulmonary hypertension |
1 (3) |
|
Age at start of Iloprost (h) |
26 (5-104) |
|
Minimum dose of Iloprost, ng/kg/min |
1 (0.5-3) |
|
Maximum dose of Iloprost, ng/kg/min |
4 (2.0-10) |
|
Duration of Iloprost, h |
97 (11-480) |
|
All values median (range), unless specified. |
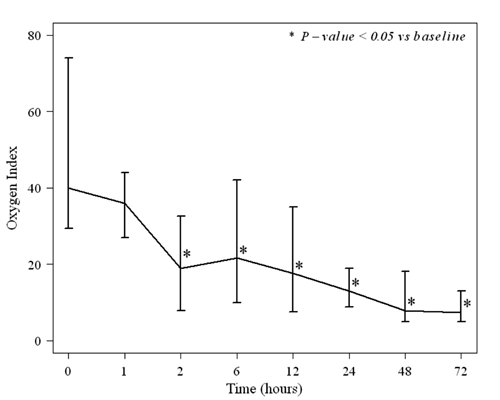
The medians (ranges) of OI, PaO2 and SpO2 before
treatment were 40 (21-101), 35 (16-210) and 79 (20-99), respectively.
IVI induced significant improvement (P<0.05) in OI, PaO2 and SpO2
compared with the baseline at 2 hours and thereafter following
initiation of treatment (Fig. 1a). The median
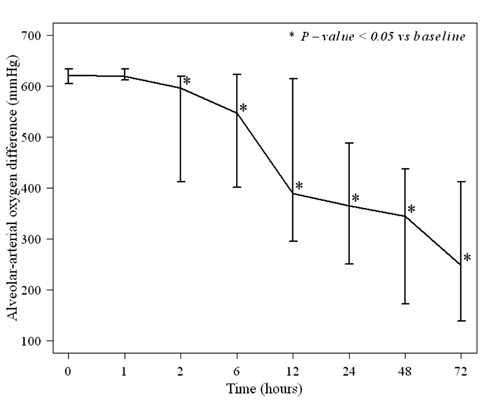
(range) AaDO 2 decreased with
statistical significance from 621 (605-633) to 597 (415-619) mmHg after
2 hours of infusion (P=0.02) (Fig. 1b).
Twenty-two (66.8%) infants were responders and 11 were non-responders.
The mortality rate was 15.2% and all of them initially had severe
hypoxemia with a median OI of 53.6. Among the mortality cases, 3 infants
(3/5, 60%) were referred from other hospitals. Five infants (5/33,
15.2%) demonstrated pneumothorax and three of these infants died.
Table II shows comparison of various characteristics between the
non-responder group and the responder group.
 |
| (a) |
(b) |
|
Fig. 1 (a) Oxygen index and (b)
Alveolar-arterial oxygen difference of neonates with PPHN before
and after intravenous iloprost administration.
|
TABLE II Risk Factors of the Responder of Iloprost-treated Severe Pphn
|
Responders
(n = 22) |
Non-
responders |
|
|
(n = 11) |
|
Birth weight, g |
3,185.4
|
2,998.7
|
|
(453.7) |
(660.8) |
|
Sex, male, n (%) |
15 (68.2) |
7 (63.6) |
|
Gestational age, wk |
38.6 (2.6) |
38.8 (3.8) |
|
#Age at start‡, h
|
26 (24, 35) |
30 (13, 39.2) |
|
#Minimum dosage‡ ng/kg/min
|
1 (0.5, 1) |
1 (1, 1.5) |
|
#Maximum dosage‡, ng/kg/min |
3.5 (2, 4) |
5 (3.5, 5.2) |
|
#Duration‡, h |
108 |
111 |
|
(37.5, 167.8) |
(40.2, 135) |
|
AaDO2 before treatment
|
614 (22.7) |
603 (74.6) |
|
Oxygen index before treatment |
47.6 (28.5) |
53.6 (27.3) |
|
Tension pneumothorax, n(%) |
3 (13) |
2 (16.6) |
|
Duration on HFOV, d |
9.2 (7.2) |
13.7 (17.5) |
|
Duration on conventional
|
|
|
|
ventilator, d |
8.7 (6.3) |
8.2 (10.9) |
|
Duration of hospitalization, d |
27.3 (17.1) |
18.9 (14.1) |
|
$Death, n(%) |
1 (2.1) |
4 (36.4) |
|
Cause of PPHN, n(%) |
|
|
|
Meconium aspiration syndrome |
12 (54.5) |
6 (54.5) |
|
Pneumonia |
8 (36.4) |
4 (36.4) |
|
Sepsis
|
2 (9.1) |
0 (0) |
|
Idiopathic PHN |
0 (0) |
1 (9.1) |
|
#Median (range);Other values in mean (SD); $P=0.01;
‡Iloprost; PPHN- Persistent pulmonary hypertension of
newborn. |
Before starting IVI, 22 (66.7%), 8 (24.2%), 2 (6.1%),
3 (9.1%) infants needed dopamine, dobutamine, epinephrine and
norepinephrine, respectively, to provide blood pressure support. During
IVI treatment, 32 (96.9%), 22 (66.7%), 11 (33.3%) and 17 (51.5%) infants
needed dopamine, dobutamine, epinephrine and norepinephrine,
respectively. There was a statistically significant increase in the
number of infants who needed inotropic medications after the start of
IVI treatment (P<0.01). After starting IVI, 21 (65.6%) infants
needed any new inotrope, 12 (57.1%), 6 (28.6%), 3 (14.3%) of infants
needed an additional 1, 2 and 3 more inotropic medications,
respectively. The median (range) time interval between starting IVI and
the need for any new inotrope was 13 (1-39) hours. During IVI treatment,
only one (3%) infant never needed a volume expander. Before starting
IVI, 27 (81.8%) infants needed a volume expander and after IVI
administration 19 (57.6%) infants needed a volume expander to provide
normal blood pressure. There was no statistically significant difference
in the number of infants who needed a volume expander after IVI
treatment (P=0.17). The median (range) time interval between
starting IVI and the need for a volume expander was 16 (2-64) hours.
There were no statistically significant differences of blood pressure
and heart rate before and after IVI treatment under inotropic
medications and volume expander therapies (P>0.05). Five
surviving infants (5/28, 17.9%) were diagnosed only as mild BPD. All of
the surviving infants had a normal hearing screening test at discharge
and no infant discharged home with oxygen support. No other
complications such as fever, facial flushing, cholestasis jaundice,
renal insufficiency, neonatal seizure or abnormal heart disease were
observed. Cranial ultrasonography was performed in 26 of 28 surviving
infants, and was reported as severe intraventricular hemorrhage in one
infant.
Discussion
Iloprost is a synthetic analogue of the natural
prostacyclin (PGI2) with a
plasma half-life of 20–30 minutes. Iloprost has been shown to decrease
pulmonary vascular resistance by inhibiting adenylate cyclase, thus
increasing the intracellular cyclic adenosine monophosphate (cAMP)
level. Furthermore, iloprost has been linked to rapid decreases in
atrial natriuretic peptide and cyclic guanosine monophosphate (cGMP)
levels and increased pulmonary clearance of big endothelin-1 in
pulmonary hypertension patients. These mechanisms are thought to be
involved in the vasodilatory actions of iloprost [14,15]. Continuous
intravenous infusion is more effective than inhaled iloprost [6];
however, no specific dosage is available for the neonatal age group. The
dosage and interval usage in this study were derived from children with
pulmonary hypertension secondary to congenital heart disease [16,17].
Other doses are also reported in literature [8,9]. The side effect of
inhaled iloprost was severe bronchoconstriction in two patients without
prior history of lung disease [18].
Iloprost affects vascular tone by mediating the
incremental levels of cAMP. This effect is complementary to the
increased cGMP levels produced by sildenafil. Because prostacyclin and
sildenafil have two different second messenger systems, their
simultaneous usage is likely to produce vasodilatation when single drug
therapy fails [14]. The high mortality rate of IVI in combination with
sildenafil in this study could be from the selection of severe cases
with unresponsiveness from IVI. Oral beraprost sodium, an oral PGI 2
analog, was reported as an adjunctive therapy in infants with PPHN [25].
Oral sildenafil and beraprost sodium could be an adjunctive therapy with
IVI. PPHN has a significantly wide spectrum of severity. The initial OI
in the infants enrolled reflects, at least in part, the degree of
illness severity and the high risk for mortality in these infants,
particularly without iNO and ECMO. The median OI before the start of
treatment in this study was higher than in the other studies in PPHN
infants with intravenous sildenafil (27.7) [19] and intravenous
milrinone with iNO (28.1) [22]. No infant with mild or moderate PPHN was
entered into this study. Furthermore, several studies in children and
adults with pulmonary hypertension secondary to heart disease have shown
that IVI can affect systemic hypotension by dilatation of arterioles and
venules [16,17]. In this study, all infants developed systemic
hypotension that required one or more inotropic medications or a volume
expander to provide blood pressure support which is different than
studies of intravenous sildenafil [23] or intravenous milrinone with iNO
[22].
This study had several limitations. This was a
retrospective study with a small number of patients limited to the
enrollment of PPHN infants diagnosed by echocardiogram and a lack of
complete long term follow-up visits for growth and neurodevelopmental
outcomes. As the study did not have a control group, the effect of
confounders need to be considered. Further, clinical pharmacokinetic
studies and optimal dosing on large neonatal populations using
randomized clinical studies are warranted to study IVI in neonates with
severe PPHN to evaluate the safety, efficacy and long-term outcomes
after treatment with IVI, both in areas with and without widespread
availability of alternative therapies like iNO. Our preliminary data
indicate that IVI can improve oxygenation and could be an adjunctive
therapy for PPHN in situations where iNO or ECMO is not available.
Acknowledgments: The authors thank Professor Don
McNeil and Ms. Nannapat Pruphetkaew (Epidemiology Unit, Faculty of
Medicine, Prince of Songkla University, Thailand) for the statistical
analysis and Mr. Glenn Shingledecker (Office of International Affairs,
Faculty of Medicine, Prince of Songkla University, Thailand) and
Associate Professor Pracha Nuntnarumit (Faculty of Medicine, Ramathibodi
University, Thailand) for editing the manuscript.
Contributors: WJ: designed the study, collected
data, drafted the paper, conducted the laboratory tests, analyzed the
data, wrote the manuscript and revised the manuscript for important
intellectual content. She will act as guarantor of the study; AT, GM, PC
and SD: drafted of the manuscript. The final manuscript was approved by
all authors.
Funding: None; Competing interests: None
stated.
|
What is Already Known?
• The standard treatment of severe pulmonary
hypertension of the newborn is inhaled nitric oxide (iNO).
What This Study Adds?
• Intravenous iloprost continuous infusion
could be a promising therapy for severe pulmonary hypertension
of the newborn as an adjunctive therapy in case iNO or ECMO is
not available.
|
References
1. Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR,
Korones SB, Stevenson DK, et al. Persistent pulmonary
hypertension of the newborn in the era before nitric oxide: practice
variation and outcomes. Pediatrics. 2000; 105:14-20.
2. Berti A, Janes A, Furlan R, Macagno F. High
prevalence of minor neurologic deficits in a long-term
neurodevelopmental follow-up of children with severe persistent
pulmonary hypertension of the newborn: a cohort study. Ital J Pediatr.
2010;36:45.
3. Clark RH, Kueser TJ, Walker MW, Southgate WM,
Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for
persistent pulmonary hypertension of the newborn. Clinical Inhaled
Nitric Oxide Research Group. N Engl J Med. 2000;342:469-74.
4. Krug S, Sablotzki A, Hammerschmidt S, Wirtz H,
Seyfarth HJ. Inhaled iloprost for the control of pulmonary hypertension.
Vasc Health Risk Manag. 2009;5:465-74.
5. Hoeper MM, Spiekerkoetter E, Westerkamp V, Gatzke
R, Fabel H. Intravenous iloprost for treatment failure of aerosolised
iloprost in pulmonary arterial hypertension. Eur Respir J.
2002;20:339-43.
6. Machherndl S, Kneussl M, Baumgartner H, Schneider
B, Petkov V, Schenk P, et al. Long-term treatment of pulmonary
hypertension with aerosolized iloprost. Eur Respir J. 2001;17:8-13.
7. De Luca D, Zecca E, Piastra M, Romagnoli C.
Iloprost as ‘rescue’ therapy for pulmonary hypertension of the neonate.
Paediatr Anaesth. 2007;17:394-5.
8. Eifinger F, Sreeram N, Mehler K, Huenseler C,
Kribs A, Roth B. Aerosolized iloprost in the treatment of pulmonary
hypertension in extremely preterm infants: a pilot study. Klin Padiatr.
2008;220:66-9.
9. Chotigeat U, Jaratwashirakul S. Inhaled iloprost
for severe persistent pulmonary hypertension of the newborn. J Med Assoc
Thai. 2007;90:167-70.
10. Greenough A, Khetriwal B. Pulmonary hypertension
in the newborn. Paediatr Respir Rev. 2005;6:111-6.
11. Development of Audit Measures and Guideline for
Good Practice in the Management of Neonatal Respiratory Distress
Syndrome. Report of a Joint Working Group of the British Association of
Perinatal Medicine and the Research Unit of the Royal College of
Physicians. Arch Dis Child 1992;67(10 Spec No):1221-7.
12. Jobe AH, Bancalari E. Bronchopulmonary dysplasia.
Am J Respir Crit Care Med. 2001;163:1723-9.
13. Volpe JJ. Intracranial hemorrhage: Germinal
matrix hemorrhage. In: Neurology of the Newborn. 5th ed.
Philadelphia: Elsevier; 2008:403-63.
14. Goldsmith DR, Wagstaff AJ. Inhaled iloprost: in
primary pulmonary hypertension. Drugs. 2004;64:763-73.
15. Herrera Torres R, Concha Gonzalez EP, Holberto
Castillo J. Sildenafil oral como alternativa en el tratamiento de recién
nacidos con hipertensión pulmonar persistente. Rev Mex Pediatr.
2006;73:159,63.
16. Tissot C, Beghetti M. Review of inhaled iloprost
for the control of pulmonary artery hypertension in children. Vasc
Health Risk Manag. 2009;5:325-31.
17. Hallioglu O, Dilber E, Celiker A. Comparison of
acute hemodynamic effects of aerosolized and intravenous iloprost in
secondary pulmonary hypertension in children with congenital heart
disease. Am J Cardiol. 2003;92:1007-9.
18. Ivy DD, Doran AK, Smith KJ, Mallory GB, Jr.,
Beghetti M, Barst RJ, et al. Short- and long-term effects of
inhaled iloprost therapy in children with pulmonary arterial
hypertension. J Am Coll Cardiol. 2008;51:161-9.
19. Vargas-Origel A, Gómez-Rodríguez G, Aldana-Valenzuela
C, Vela-Huerta MM, Alarcón-Santos SB, Amador-Licona N. The use of
sildenafil in persistent pulmonary hypertension of the newborn. Am J
Perinatol. 2010;27:225,225-30.
20. Baquero H, Soliz A, Neira F, Venegas ME, Sola A.
Oral sildenafil in infants with persistent pulmonary hypertension of the
newborn: a pilot randomized blinded study. Pediatrics. 2006;117:1077-83.
21. Mohamed WA, Ismail M. A randomized, double-blind,
placebo-controlled, prospective study of bosentan for the treatment of
persistent pulmonary hypertension of the newborn. J Perinatol.
2012;32:608-13.
22. McNamara PJ, Laique F, Muang-In S, Whyte HE.
Milrinone improves oxygenation in neonates with severe persistent
pulmonary hypertension of the newborn. J Crit Care. 2006;21:217-22.
23. Steinhorn RH, Kinsella JP, Pierce C, Butrous G,
Dilleen M, Oakes M, et al. Intravenous sildenafil in the
treatment of neonates with persistent pulmonary hypertension. J Pediatr.
2009;155:841-7 e1.
24. Ahsman MJ, Witjes BC, Wildschut ED, Sluiter I,
Vulto AG, Tibboel D, et al. Sildenafil exposure in neonates with
pulmonary hypertension after administration via a nasogastric tube. Arch
Dis Child Fetal Neonatal Ed. 2010;95:F109-14.
25. Nakwan N, Wannaro J. Persistent pulmonary
hypertension of the newborn successfully treated with beraprost sodium:
a retrospective chart review. Neonatology. 2011;99:32-7.
|
|
|
 |
|

