|
S.N. Singh
A.K. Patwari
R. Dutta
Neelam Taneja
V.K. Anand
From the Departments of Pediatrics and Microbiology, Lady Hardinge Medical College and Associated Kalwati Saran Children's Hospital, New Delhi 110001, India.
Reprint requests: Dr. A.K. Patwari, Professor of Pe- diatrics, Lady Hardinge Medical College and Associated Kalawati Saran Children's Hospital, New Delhi 110
001, India.
Manuscript Received: January 16, 1998; Initial review completed: March 24, 1998; Revision Accepted: April 22, 1998.
Infection of central nervous system (CNS) in humans with free living amebae is uncommon. Among many different genera of amebae Naegleria and Acanthamoeba are pathogenic to CNS. Primary amebic meningoencephalitis. (PAM) caused by these amebae was first reported in 1965 by Fowler and Carter in Australia(l). There are two distinct clinical syndromes caused by free living amebae affecting CNS. PAM, caused by Naegleria fowleri, occurs in healthy children and young adults who usually have been recently swimming in fresh water. Granulomatous
amebic encephalitis (GAE), caused by Acanthamoeba, is a subacute opportunistic infection. More than 140 cases of PAM caused by Naegleria have been reported from all over the world and mostly had been fatal(2). This report describes a case of Naegleria meningitis successfully treated with amphotericin Band rifampicin.
Case report
An eight-year-old boy was admitted in October 1996 with 2 days history of high
grade fever, vomiting and headache. On examination he was febrile (102o F), conscious and neck rigidity and kernig's sign were positive, without any localizing signs. Optic fundi and systemic examination were normal. Cerebrospinal fluid (CSF) examinaion revealed sheets of polymorphs with protein concentration of 160 mg/ dl and glucose 30 mg/ dl. Gram staining and bacterial culture of CSF
were negative. Treatment was started with first. line antibiotics (crystalline penicillin and chloramphenicol) and other supportive measures. On 3rd day, headache and vomiting subsided and fever came down to 90-100' F. On 10th day the patient's temperature shot up again to 102o F and repeat CSF
examination revealed 450 cells/mm3 with predominance of polymorphs. CSF protein and sugar were 84 mg/ dl and 40 mg/ dl, respectively. Gram staining and culture of CSF were negative. Workup for tuberculosis (tuber- culin test, X-ray chest and AFB in CSF) was negative. The antibiotics were changed to ceftazidime and ampicillin. On 21st day the patient continued to have fever, developed headache and vomiting, and optic fundi suggested papilledema. Lumbar puncture was performed with due caution and the CSF sample was transported fresh, immediately to the laboratory in a plain sterile vial. CSF examination showed 1600 cells/ mm3 with predominance of neutrophils, protein was 97 mg/ dl and sugar was 28 mg/ dl. Gram staining and bacterial culture of CSF were again negative. However, cells
with ameboid movement were observed in wet mount of the CSF which was con- firmed by repeat lumbar puncture and
Naegleria
trophozites were reported in fresh
CSF specimen
(Fig.
1). CSF was cultured on non-nutrient agar plates which were precoated with a suspension of Escherechia
coli
prepared in Page Ameba Saline from an
18-24 hour old culture of Escherechia
coli.
The CSF was placed in the centre of the plate and was incubated at 37oC.
The plates were observed daily for 7 days under low power (10X) objective of a microscope, for the amebae. CSF culture also grew Naegleria. Patient did not reveal any history of swimming. Nasal examination was normal but nasal smear revealed motile trophozoites of Naegleria and culture from nasal scrappirigs grew Naegleria. CT head revealed findings suggestive of basal meningitis with communicating hydrocephalus. Treatment was started with amphotericin B intravenously (1 mg/kg/ day) and oral rifampicin (10 mg/kg/day in 3 divided doses). Amphotericin B was ad- ministered intrathecally once only as the patient developed severe bachache
following the injection and refused further injection. Injection hydrocortisone and chlorpheniramine was given for rigors during the amphotericin-B infusions. The patient's condition stabilized within the next 3 days of antinaegleria therapy, and improved during next one month of hospitalization. Therapy was continued for 4 weeks till CSF became clear of Naegleria trophozoites. At discharge, patient was afebrile with no neurologic
deficit. Microscopic examination and culture of nasal scrappings were negative for Naegleria. During 6 months follow up, the patient was found to. be asymptomatic.
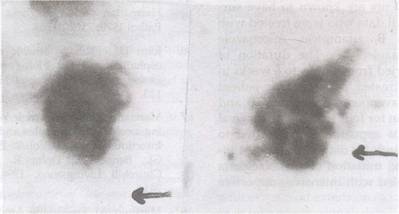 |
|
Fig.
1. Wet mount of CSF
showing (a) trophozoite (arrow showing lobopodia) and (b)
intermediate stage of Naegleria species. |
Discussion
PAM is still unfamiliar to many clinicians, pathologists and microbiologists. Most human infections with Naegleria have been associated with swimming in warm water, but other reported sources of infection include tap water(3), hot baths(4) and air(5). Our case presumably acquired the infection from air. Subclinical infection due to Naegleria is possible in healthy individuals when these amebae colonise the nose and throat(6). The olfactory neuroepithelium is the anatomic site
of invasion by the Naegleria. PAM is a disease with an abrupt onset and a fulminant
course. No distinctive clinical feature differentiates PAM from acute bacterial meningoencephalitis on clinical grounds. After an incubation period of 2 to 15 days, high fever, anorexia, vomiting, headache, meningismus
and changes in mental status may occur. The patient may rapidly progress to coma
usually without ever developing focal neurologic signs. Most patients are dead within a week after the on-set of the illness(7). The peripheral blood white cell count is usually elevated, with a predominance of neutrophils. CSF picture resembles those of acute bacterial meningitis with hemorrhagic CSF at times(2,7).
The diagnosis. of PAM can be made with certainty only by demonstration of motile Naegleria trophozoites in wet mount of CSF or in brain. Antibodies to Naegleria sp. have been detected in normal persons and serology is not useful in the diagnosis in acute stage(6). Head computed tomographic (CT) scan in one patient with PAM
showed diffuse contrast enhancement of the grey matter and obliterated ambiens, interpeduncular and quadrigeminal cisterns(8). The prognosis is uniformly poor. Only six patients are known to have survived PAM till data who were treated with amphotericin B, rifampicin, miconazole and ketQconazole(9-13). The duration of treatment varied from 9 days to 6 weeks in successfully treated patients. Our patient received intravenous amphotericin B and
oral rifampicin for' four weeks. The survival of our patient appeared to be
related to continued search for diagnosis, prompt intervention and initiation of anti Naegleria therapy coupled with intensive supportive. care.
In conclusion, although PAM is rare and has poor prognosis, it should be considered in any patient who has purulent
meningitis without evidence of bacteria by Gram stain, antigen detection and culture. It is very important to examine a wet mount of CSF for amebic trophozoites.
|
1.
Fowler M, Carter RF. Acute pyogenic meningitis probably due to Acanthamoeba sp: A preliminary report. Br Med J 1965; 2: 740-742.
2.
Pearl MA, Visvesvara GS, Martinez AJ, Theodore FH, Dagget PM, Sawyer TK. Naegleria and Acanthamoeba infection: Re- view. Rev Infect Dis 1990; 12: 490-513.
3.
Warhurst DC, Mann PG. Primary amaebic meningoencephalitis in Bath Spa, England. In: Proceeding of the 2nd Inter- national Conference on the Biology and Pathogenicity of Small Free Living Amebae. Florida, Gainesville University of Florida, 1980; p 55.
4.
Singh BN, Das SR. Occurrence of Pathogenic Naegleria aerobia, Hartmannella culbertsoni and H. rhysodes in sewage sludge sample of Lucknow. Curr Sci 1972; 41: 277-281.
5.
Lawande RV, Abraham SN, John I, Egler LJ. Recovery of soil amebas from nasal passages of children during the dusty Harmattan period in Zaria. Am
J
Clin PathoI1979;71:201-203.
6. John DT. Primary amebic meningoencephalitis and the biology of Naegleria
fowleri. Ann Rev Microbiol 1982; 36: 101-123.
7.
Marciano-Cabral F, Petri Jr WA. Free-living ameboe. In: Principles and Practice of Infectious Diseases, Vol. 2. Eds Mandell GL, Bennet JE, Dolin R. New York, Churchill Livingstone, 1994; pp 2408- 2414.
8.
Martinez AJ. Free living Amoebas: Natural History, Prevention, Diagnosis, Pathology and Treatment of Disease. Boca Raton, FL, CRC Press, 1985.
9.
Seidel JS, Harmatz P, Visvesvara GS. Successful treatment of PAM. N Engl
J
Med
1982; 306:
346-348.
10.
Brown RL. Successful treatment of PAM. Arch Intern Med 1991; 151: 1201-1202.
11. Anderson K, Jamieson A. Primary amebic meningoencephalitis. Lancet 1972; 1: 902- 903.
12.
Poungvarn N, Jariya P. The fifth nonlethal case of PAM.
J
Med Assoc Thia 1991; 74: 112-115.
13.
Wang A, Kay R, Poon WS. Successful treatment. of amebic meningoencephalitis in a Chinese living in Hong Kong. Clin Neural Neurosurg 1993; 95: 249- 252.
|
