afety and efficacy are amongst the key criteria
prior to the licensing and marketing authorization of vaccines by
national regulatory authorities across the world. The principle that
benefits should outweigh the (small) risk associated with vaccine
administration is upheld. The safety assessment of vaccines requires
continuous surveillance of the risks associated with vaccine
administration, which in turn requires – among other mechanisms – a
functioning system to record and report any health event that may follow
vaccination. In India, the first documented investigation of an adverse
event after vaccination goes back to as early as the year 1902 [1].
Decades later, a formal monitoring mechanism to record and report
adverse events following vaccination as part of national program was
outlined at the launch of Universal Immunization Program (UIP) in India
in 1985 [2]. Thirty years since then, the Adverse Events Following
Immunization (AEFI) surveillance system in India has come a long way,
and now there are mechanisms of case-based surveillance and
investigation of serious AEFIs, while non-serious (or minor) AEFIs are
reported and documented through routine and aggregate reporting
mechanism [3].
In the last two decades, the vaccines against a
number of additional disease-causing agents have been licensed in
countries with different income levels. While the use of newer vaccines
is widespread in high income countries, the availability of these
vaccines in majority of low- and middle-income countries (LMICs) is
restricted to physicians in the private sector offering vaccination
services. Moreover, the availability and accessibility of information
about vaccines on the World Wide Web has not only led to the increased
demand for vaccination but also for information on the safety of
vaccines. What is applicable to vaccines as one of the health
interventions is also applicable for any other health intervention. The
demand for information on any aspect of vaccination (including on the
safety) is invariably higher than the health systems in LMICs are
prepared and capable to generate. In this background, innovative
approaches to gather additional information on new health interventions
have to be explored and optimally utilized.
This issue of Indian Pediatrics publishes a
study by Kompithra, et al. [4], that compares the common
illnesses before and after vaccination through ‘risk interval approach’.
The study has been conducted at a tertiary care level health facility in
private sector in a state in Southern part of India [4]. A key finding
reported is that with the exception of fever, rates of other common
childhood illnesses reported before vaccination were higher than in the
post-vaccination period. From the literature cited in this study, one
can notice that there may not be many studies (at least published) on
this topic from India. This is probably one of the first published
studies to systematically compare the common illnesses before and after
vaccine adminis-tration (often termed non-serious AEFIs) from Indian
settings. Though, there are methodological limitations in this study as
also mentioned by the authors, these should be considered as learning
points by researchers to design better studies in future. One of the
strengths of this study is that the comparative analysis presented by
the authors is not possible through existing aggregate reporting
mechanisms for non-serious AEFIs in India.
This study could be an appropriate starting point for
more research to generate comparable data from public and private sector
and different geographical locations in the country. Such data could
prove extremely useful for decision-making on the introduction of newer
vaccines as often requested by technical committees such as National
Immunization Technical Advisory Groups, prior to the recommendations on
the inclusion (or not) of newer vaccines in the national immunization
programs of a country. The newer vaccines become available in the
private sector soon after licensing by regulatory body of a country. In
LMICs, there is average time lag of 10-15 years before such vaccines
become part of public vaccination program. In this context, evidence
generated through sufficiently robust scientific methodology (on safety
and other aspects) could be a valuable source of additional data for
decision-making. For example, in India private sector provides
vaccination to nearly 10-20% of approximately 125 million under-five
children. Therefore, data from even a fraction of this cohort over
period of a few years could be a large evidence base.
The information generated by this study is valuable.
However, such studies should not be the only source of data, and there
is a need for systematic institutional mechanisms for long-term evidence
generation. The health systems and surveillance mechanisms in LMICs
should be strengthened by specific focus upon areas such as improving
immunization service delivery to additional populations, providing
additional financial resources, capacity building of staff at various
levels in data collection, reporting and analysis, preparing conducive
and supportive policies, and providing leadership at all levels. Such
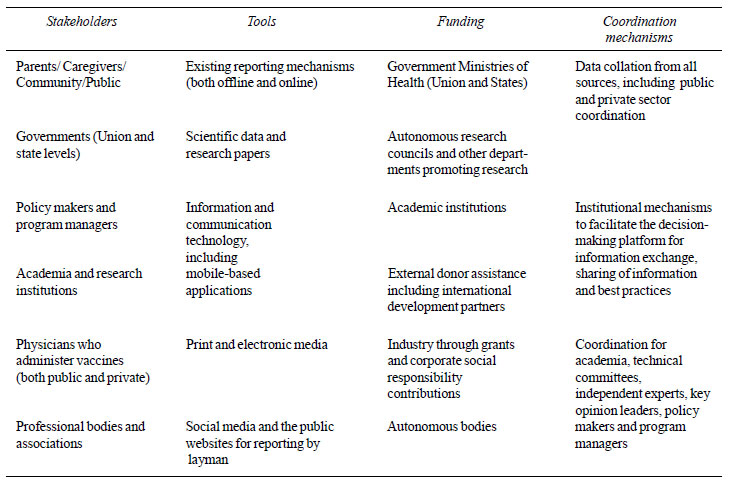
efforts require involvement of wider numbers of stakeholders. There is a
need for working through a comprehensive framework (Table I)
on the inclusion of stakeholders, with possible tools, funding sources
and coordinating mechanisms, to streamline the dialogue and strengthen
the health systems in more coordinated ways to improve immunization
services in LMICs.
|
TABLE I Stakeholder Engagement for
Improving Vaccine Safety
|
 |
The health systems in LMICs need to be prepared to
generate information to assist policy makers in all relevant aspects of
decision-making components. Every opportunity, which comes our way,
should be used to reflect upon the need for institutional mechanisms and
health systems for strengthening the evidence-based policy decision
making. It is up to the stakeholders to decide together on how quickly
they would like to act upon what needs to be done at the earliest
possible.
1. Lahariya C. A brief history of vaccines &
vaccination in India. Indian J Med Res. 2014;139:491-511.
2. Sokhey J. Adverse events following
immunization:1990. Indian Pediatr. 1991;28:593-607.
3. Government of India. Adverse Event Following
Immunization: Surveillance and Response Operational Guidelines 2015.
Ministry of Health and Family Welfare, Government of India, Nirman
Bhawan, New Delhi. 2015.
4. Kompithra RZ, Sarkar R, Mathew LG, Muliyil J, Kang
G. Study of common illnesses before and after vaccination: A
risk-interval approach. Indian Pediatr. 2015;52:933-8.

