Subclinical hypothyroidism (SCH) is a biochemical
condition characterized by serum levels of Thyroid Stimulating Hormone
(TSH) above the statistically defined upper limit of reference range,
with normal concentration of thyroid hormones, and without clinical
features of hypothyroidism [1]. SCH is a common disorder with a
prevalence of 1-10% in adults and about 2% in children; epidemiological
studies concerning childhood and adolescence are scarce [2-4]. SCH is
mostly detected incidentally as patients exhibit few or no signs of
thyroid dysfunction. The abnormalities most frequently associated in the
pediatric population are goiter, poor school performance, weight gain,
increased cholesterol levels, impaired growth velocity, anemia,
excessive sleepiness, weakness, and impaired psychomotor and cognitive
development [4,5].
Normal TSH Level
TSH is secreted in a pulsatile manner and shows
diurnal variation. The levels may vary based on the time of sampling as
well as its relation to food [6]. Most of the commercially available
kits use third generation TSH assays like radioimmunoassay,
chemiluminescence or electrochemiluminescence method. There is no
biolo-gical reference range derived from these kits based on studies in
pediatric population in India. The reference range given in the kit by
the manufacturers of these assays vary. TSH above the laboratory
reference ranges are considered abnormal by most pediatricians. These
factors add to the difficulty in interpreting the TSH values and in
decision-making for the clinician [7]. Two large population studies from
India by Marwaha, et al. [8,9] reported normograms for TSH in
Indian children. In study amongst children 5-16 yrs, the mean and 97th
percentile for TSH (radioimmunoassay method) was, 3.17 and 7.5,
respectively. This gives us a range of 1.33-5.01 mIU/L as normal values
for our population. Almost 12% of the reference population had TSH
values above the normal range provided by the test kit manufacturer.
Such patients need long-term follow up for development of overt
hypothyroidism.
Etiology
SCH is most commonly (50-80% of cases) caused by
chronic autoimmune thryoiditis, which is typically characterized by high
titers of thyroid peroxidase antibodies, thyroglobulin antibodies and
rarely TSH-receptor blocking antibodies [10]. There are many
causes of potentially reversible/irreversible subclinical hypo-thyroidism
[11] (Box I). Non-thyroidal causes include diabetes
mellitus, cystic fibrosis, celiac disease, and chronic renal failure
[12].
| BOX I
Differential Diagnosis of Elevated TSH After Infancy
|
|
Reversible
Autoimmune thyroiditis
Recovering from acute illness
Recovering from subacute
thyroiditis
Antithyroid drugs
Simple obesity
Cortisol deficiency
Laboratory error
Irreversible
Autoimmune thyroiditis
Thyroid dysgenesis
Subtotal/hemi thyroidectomy
Neck radiotherapy,
Reidel’s thyroiditis
|
Mutations in several proteins involved in TSH action
have been demonstrated. Loss of function mutations in the TSH receptor
gene have been demonstrated [13,14]. Dual oxidase 2 (DUOX2),
phosphodiesterase 8B and thyroidperoxidase mutations have also been
reported as causes of mild elevations of TSH [15-17]. Congenital
conditions are commonly associated with SCH. SCH is also associated with
Down syndrome; present in up to 32% of these patients. Anti-thyroid
antibodies were not more likely to be found in this group than in
patients with a normal TSH [18]. Almost one-third patients with William
syndrome also have SCH with negative anti-thyroid antibodies [19].
Abnormal sialylation of the carbohydrate moiety of
TSH with resultant reduced metabolic clearance may also contribute to
elevated TSH in occasional cases of hypothyroidism [20].
Epidemiology
Large scale population studies focussing on the
prevalence of SCH among children, especially from India are limited.
With the difficulty in defining normal TSH, the prevalence reported in
different studies may vary depending on the cut-off value. The sample
selection in many of the studies is strictly not representative of the
general pediatric population. In some follow-up studies, a mildly
elevated TSH has been documented to normalize after few months
[21]. Persistently elevated TSH over a period of time may be the best
indicator to assess the true prevalence of SCH in the pediatric
population.
Marwaha, et al. [22] conducted a
large nationwide survey on the thyroid status after 2 decades of salt
iodization in India. The prevalence of subclinical and overt
hypothyroidism was 6.1% and 0.4%, respectively among the study
population (total population of 38961 children). TSH elevation was found
more common among children with goiter. The prevalence of goitre among
the studied population was 15.5%, much above 5% prescribed by WHO.
Further, thyroid autoimmunity, as defined by positive thyroperoxidase
antibody titers, was observed in 3.6% of the study population and was
more common among girls. In another study from Chandigarh, India, goiter
prevalence was 15.1% and that of SCH was 2.6%. The population studied
was iodine sufficient in that study with prevalence of autoimmunity not
significantly different from the controls [23]. A study from USA, done
primarily to assess cognitive parameters among adolescents with thyroid
disorders, the prevalence of SCH was 1.7% [4]. Lazar, et al.[21],
in a retrospective analysis from an insurance-based large database of
children between 6 months to 16 years of age, reported a prevalence of
elevated TSH (5.5-10.0 mIU/6) to be 2.9% [21]. Transiently elevated TSH
may occasionally be diagnosed as part of newborn screening program. In a
large series from China, the incidence was 1 in 8809 neonates
[24]. The TSH elevation was treated with thyroxine replacement,
considering its critical role in neurocognitive development, with a
favourable outcome at 2-3 years follow up. Long term follow-up of these
children was not available to know whether the TSH rise was transient or
persisted beyond 3 years of age. SCH is observed more commonly in obese
children when compared with normal weight controls; excess adipose
tissue is hypothesized to signal elevation in TSH [25].
Children with Down syndrome are at increased risk –
upto 28 times the normal population – for hypothyroidism.
Autoimmune predisposition or dysgenesis may contribute to thyroid
dysfunction among children with this chromosomal anomaly [26]. In this
setting, SCH may warrant treatment as the progression to overt
hypothyroidism is more likely.
Type 1 diabetes predisposes children to thyroid
dysfunction. In a study by Soliman, et al. [27], the prevalence
of SCH in children (mean age 10 yrs) with type I diabetes was 11.2%.
Other conditions which may be associated with elevated risk for SCH
include antiepileptic drug usage and celiac disease.
Clinical Issues
Most patients with SCH exhibit few or no signs or
symptoms of hypothyroidism. It has been suggested that some patients
have functional, clinical, or biochemical manifestations of
hypothyroidism that are more common than age-matched controls [28].
Goiter is the most common manifestation [12]. The abnormalities found
most commonly in the pediatric population include weight gain, increased
cholesterol levels, impaired growth velocity, anemia, sleepiness,
weakness, and impaired psychomotor and cognitive development [5].
Natural Progression of SCH and Effects of
Intervention
There are very few prospective studies evaluating the
natural progression of SCH in pediatric age group (Table I).
In a study from India, a cohort of 32 children with SCH and autoimmune
thyroiditis (AIT) and goiter were followed. Development of overt
hypothyroidism (12.5% in this cohort) was insidious, and was not
accompanied by symptoms and signs [29]. In a larger study on 323
children with either Hashimoto or idiopathic SCH followed up for 3
years, 13.5% of SCH developed overt hypothyroidism. The study could not
detect predictive factors for progression of SCH to overt hypothyroidism
in idiopathic SCH [30]. Wasniewska, et al. [31]
followed up 92 patients with idiopathic SCH over 2 years, and none of
them developed overt hypothyroidism. Lazar, et al. [21] studied
3510 patients with SCH over 5 years and showed that 73.6% of them
normalized TSH. Elevated antibodies (thyroid peroxidise (TPOab) and
thryoglobulin antibodies (TGab)) may predict future overt hypothyroidism
and TPOab>TGab may predict impending thyroid failure in AIT [32,33].
Leonardi, et al. [35] studied 44 Italian children "false
positive" to neonatal screening for congenital hypothyroidism; 28 of
them had SCH on re-testing at 2-3 years of age. Twenty of these 28
children were treated with replacement therapy and then withdrawn from
therapy 2-3 months prior to re-evaluation. Out of the 28 children with
SCH, TSH was normal in 9 children (32%) and persistently elevated in the
remaining 19 (62%) at 4.1-6.6 yrs of age. At 7.2-9.5 yrs of age, TSH
remained normal in 9 children who previously normalized their thyroid
function, returned to normal in 5 out of 19 of the children with
previous elevated TSH and persisted above normal in remaining fourteen
childrens.
TABLE I Natural History and Progression of SCH in Pediatric Case Series
Authors
Year; Place |
Number
of patients |
Level of evidence/ |
Period of
followup
|
Key results |
Comments |
|
|
Type of study |
|
|
|
|
Radetti, et al. [32]2012; Italy |
323 |
Retrospective cross-sectional |
3 years |
13.5% of SCH developed OH |
There were no predictors in pts of SCH. |
|
Wasniewska, et al. [31]2009; Italy |
92 with SCH |
Prospective observational |
2 years |
38 normalized TSH54 remained SCH11 had increase of TSH more than
10miu/mL |
None developed OH.Natural progression in idiopathic SCH is a
progressive decrease over time of TSH in majority. |
|
Lazar, et al. [21]2009; Israel |
121052 of which 2.9% had SCH |
Prospective observational |
5 years |
In SCH group 73.6% normalizedTSH, 2% increase >10miu/mL,
and 0.03% had OH |
Female patients with >7.5miu/mL of TSH are at greater risk of
sustained raise. |
|
Gopalakrishanan, et al. [29]2008; India |
98 of which32 had SCH |
Longitudinal study |
24 months |
4/32 patients with SCH developed OH |
Important to monitor TFT. Development of OH is
insidious and may not be accompanied by symptoms and clinical
signs. |
|
Leonardi, et al. [35]2008; Italy |
44
|
Prospective observational |
8 years |
14 had SCH at end of the study. None developed OH |
Newborn false positive TSH have an increased risk of developing
SCH |
|
Radetti, et al. [30]2006; Italy |
160 of which 55 were SCHRest euthyroid |
Prospective observational |
5 years |
16/55 SCH normlaized TFT.16 remained SCH 23 had twofold rise
above the normal limit |
Presence of goitre and elevated TGAb,together with increase in
TPOab and TSH may predict future OH. At 5 yrs 50% of
all participants remained euthyroid. |
|
Zois, et al. [33]2006; Greece |
29 with AIT of which 7 had SCH |
Prospective observational |
5 years |
All 7 continued to be in SCHNone of the 29 developed OH |
TPOab>TGab increase predicted impending thyroid failure in AIT.
Thyroid hypoechogenicity seem to predict the same |
|
Jaruatanasirikul,et al. [34]2001; Thailand |
46 of which 8 had SCH |
Prospectiveobservational |
6 years |
4/8 SCH normalized TSH4/8developed OH |
No clinical or biochemical marker at baseline predicted
course of SCH |
|
|
|
|
|
Moore, et al. [36]1996; UK |
18 with SCH and AIT |
Prospective observational |
5.8 yrs |
7/18 were euthyroid10 remained SCH1 became OH |
Expectant management is recommended in majority of SCH with
minimally elevated TSH |
|
SCH- Subclinical Hypothyroidism, TFT- Thyroid function
tests, TPOab- thyroid peroxidise antibodies, TGab- thyroglobulin
antibodies, TSH- Thyroid stimulating hormone, OH- Overt
hypothyroidism, AIT- autoimmune thyroiditis. |
Effect of Treating Children with SCH
This aspect has been even less investigated and a
summary of the evidence is presented in Table II.
Wasniewska, et al. [37] compared thyroxine treated and untreated
SCH over 2 years and found no significant changes in TSH values in both
groups. Cetinkaya, et al. [38] treated 39 children with short
stature and SCH; improvement in height was significant in pre-pubertal
as compared to pubertal age group, with no progression to overt
hypothyroidism in any in the cohort. Chase, et al. [39] noted a
similar significant height increase in the pre-pubertal age group as
compared to the pubertal age group when children with SCH and type 1
diabetes were given thyroxine replacement therapy. Aijaz, et al.
[5] studied short term thyroxine replacement therapy and its effects in
neuropsychological outcome and concluded no significant change .
TABLE II Studies Reporting Effect of Replacement Therapy in Childhood SCH
|
Authors
|
Patients |
Type of study |
Follow-up |
Results |
Comments |
|
Wasniewska,
|
69 treated
|
Case control
|
2 y
|
Significant
|
TSH value changes between
|
|
et al. [37]
|
SCH vs 92
|
|
|
difference was not found |
treated and untreated groups
|
|
untreated SCH |
|
|
|
were similar. therapy is unable
|
|
|
|
|
|
to prevent the risk of further
|
|
|
|
|
|
TSH increase after treatment
|
|
|
|
|
|
withdrawal |
|
Aijaz, et al.
|
11 SCH
|
Interventional
|
91 d
|
Short term thyroxine therapy |
Thyroxine therapy showed
|
|
[5]
|
children |
|
|
showed no neuropsychological |
no positive effect on neuro-
|
|
|
|
|
benefits as compared to normal
|
psychological function in
|
|
|
|
|
population |
children with SCH |
|
Cetinkaya,
|
2067 total,
|
Interventional
|
12 mo
|
Showed improvement in |
Short stature can be associated
|
|
et al. [38]
|
39 SCH |
|
|
growth velocity; no hyper- |
with SCH. Thyroid hormone
|
|
|
|
|
thyroidism noted after |
replacement improves the
|
|
|
|
|
replacement.
|
height in such patients |
|
Chase, et al.
|
25 diabetic
|
Case control
|
2 y
|
Pre-pubertal diabetics showed |
Higher the initial TSH value
|
|
[39]
|
children
|
|
|
increased growth velocity than |
showed increased growth
|
|
with SCH |
|
|
postpubertal diabetics |
velocity
|
|
SCH- Subclinical hypothyroidism, TFT- Thyroid function
tests, TSH- Thyroid stimulating hormone, OH- Overt
hypothyroidism, AIT- autoimmune thyroiditis, TRH- thyrotrophin
releasing hormone. |
Management of SCH
Based on available literature, SCH seems to be a
benign condition which requires periodic follow-up and monitoring of
thyroid function tests. Expectant management is the norm for this
condition. Natural progression to OH does occur but lot less frequently
than expected. There appeared to be no long-term effects of untreated
SCH on growth, puberty or neuro-cognitive function; however, there is a
lack of high-quality evidence.
We propose an algorithm (Fig. 1) for
management of subclinical hypothyroidism in pediatric age group. The
first step in our setting on patients with elevated TSH, especially
below 10mIU/L, is to repeat the test on another day, preferably from
another laboratory with a different kit. SCH in adults is associated
with dyslipidemia and subtle cardiac dysfunction, with reasonable
benefit of treatment of SCH on those parameters. However, pediatric
studies focussing on the same are scarce and more research is needed on
these issues in this age group.
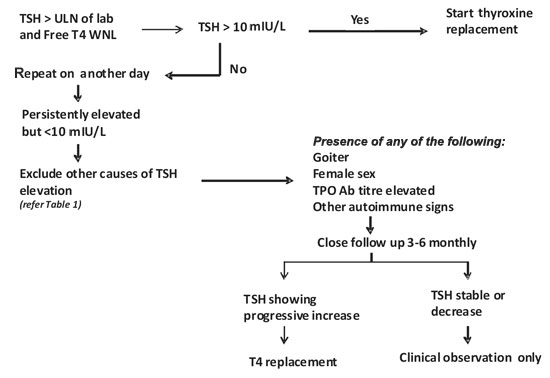 |
|
Fig.1 Approach to subclinical
hypothyroidism (SCH) in children.
|
Girls, goiter, family history of thyroid disorder,
other autoimmune problems, markedly elevated TPO titers (at least 3
times the upper limit of normal and symptoms which may correlate with
hypothyroidism are risk factors; a clinical decision to start on
thyroxine may be taken if any or combination of above is present.
Summary
SCH is a biochemical entity commonly faced by
practising pediatricians. Several factors including clinical condition
of the child and laboratory factors influencing TSH levels should be
considered while interpreting the results. Clinical decision to treat
marginal elevation in TSH should be made keeping in mind that more often
TSH normalizes without treatment if followed up over a period of time.
Even if the decision to treat the slightly elevated TSH is made, a clear
plan should be made to stop treatment and reassess after 1-2 years to
see if the treatment is required lifelong.
Contributors: Both authors searched the
literature, drafted the article and finalized the manuscript.
Funding: None; Competing interests: None
stated
References
1. Surks MI, Ortiz GH, Sawin CT. Subclinical
thyroid disease: Scientific review and guidelines for diagnosis and
management. JAMA. 2004;291:228-38.
2. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC.
The Colorado thyroid disease prevalence study. Arch Intern Med.
2000;160:526-34.
3. Paoli-Valeri M, Maman-Alvardo D, Jiménez-Lopez
V. Frequency of subclinical hypothyroidism among healthy children
and those with neurological conditions in the state of Mérida,
Venezuela. Invest Clin. 2003;44:209-18.
4. Wu T, Flowers JW, Tudiver F. Subclinical
thyroid disorders and cognitive performance among adolescents in the
United States. BMC Pediatr. 2006;6:12.
5. Aijaz NJ, Flaherty EM, Preston T.
Neurocognitive function in children with compensated hypothyroidism:
lack of short term effects on or off thyroxin. BMC Endocr Disord.
2006;6:2.
6. Scobbo RR, Vondohlen TW, Hassan M, Islam S.
Serum TSH variability in normal individuals: the influence of time
of sample collection. W V Med J. 2004;100: 138-42.
7. Sarkar R. TSH comparison between
chemiluminescence (Architect) and electrochemiluminescence (Cobas)
immunassays: An Indian population perspective. Indian J Clin Biochem.
2014;29:189-95.
8. Marwaha RK, Tandon N, Desai AK, Kanwar R,
Aggarwal R, Sastry A, et al. Reference range of thyroid
hormones in healthy school-age children: Country-wide data from
India. Clin Biochem. 2010;43:51-6.
9. Marwaha RK, Tandon N, Desai A, Kanwar
R, Grewal K, Aggarwal R, et al. Reference range of thyroid
hormones in normal Indian school-age children. Clin Endocrinol(Oxf).
2008;68:369-74.
10. Palmieri EA, Fazio S, Lombardi G. Subclinical
hypothyroidism and cardiovascular risk: A reason to treat? Treat
Endocrinol. 2004;3:233-44.
11. Papi G, Uberti ED, Betterle C. Subclinical
Hypothryoidism. Curr Opin Endocrinol Diabetes Obes. 2007;14:197-208.
12. Cooper DS. Clinical practice. Subclinical
hypothyroidism. N Engl J Med. 2001; 345:260-5.
13. Narumi S, Muroya K, Abe Y, Yasui M, Asakura
Y, Adachi M, et al. TSHR mutations as a cause of congenital
hypothyroidism in Japan: A population-based genetic epidemiology
study. J Clin Endocrinol Metab. 2009;94:1317-23.
14. Nicoletti A, Bal M, De Marco G, Baldazzi L,
Agretti P, Menabo S, et al. Thyrotropin-stimulating hormone
receptor gene analysis in pediatric patients with non-autoimmune
subclinical hypothyroidism. J Clin Endocrinol Metab.
2009;94:4187-94.
15. De Marco G, Agretti P, Montanelli, Dicosmo C,
Bagattini B, De Servi M, et al. Identification and functional
analysis of novel dual oxidase 2 (DUOX2) mutations in children with
congenital or subclinical hypothyroidism. J Clin Endocrinol Metab.
2011;96:E1335-9.
16. Grandone A, Perrone L, Cirillo G, Di Sessa A,
Corona AM, Amato A, et al. Impact of phosphodiesterase 8B
gene rs4704397 variation on thyroid homeostasis in childhood
obesity. Eur J Endocrinol. 2012;166:255-60.
17. Turkkahraman D, Alper OM, Aydin F, Yildiz A,
Pehlivanoglu S, Luleci G, et al. Final diagnosis in children
with subclinical hypothyroidism and mutation analysis of the thyroid
peroxidise gene (TPO). J Pediatr Endocrinol Metab. 2009;22:845-51.
18. Rubello D, Pozzan GB, Casara D, Girelli ME,
Boccato S, Rigon F, et al. Natural course of subclinical
hypothyroidism in Down’s syndrome: Prospective study results and
therapeutic considerations. J Endocrinol Invest. 1995;18:35-40.
19. Schaub RL, Hale DE, Rose SR. The spectrum of
thyroid abnormalities in individuals with 18q deletions. J Clin
Endocrinol Metab. 2005;90:2259-63.
20. Persani L, Borgato S, Romoli R, Asteria C,
Pizzocaro A, Beck-Peccoz P. Changes in the degree of sialylation of
carbohydrate chains modify the biological properties of circulating
thyrotropin isoforms in various physiological and pathological
states. J Clin Endocrinol Metab. 1998;83:2486-92.
21. Lazar L, Frumkin RB, Battat E, Lebenthal Y,
Phillip M, Meyerovitch J. Natural history of thyroid function tests
over 5 years in a large pediatric cohort. J Clin Endocrinol Metab.
2009;94:1678-82.
22. Marwaha RK, Tandon N, Garg MK, Desai A,
Kanwar R, Sastry A, et al. Thyroid status two decades after
salt iodization: country-wide data in school children from India.
Clin Endocrinol (Oxf). 2012;76:905-10.
23. Das S, Bhansali A, Dutta P, Aggarwal A,
Bansal MP, Garg D, et al. Persistence of goitre in the
post-iodization phase: micronutrient deficiency or thyroid
autoimmunity? Indian J Med Res. 2011;133:103-9.
24. Xiao Chen X, Feng Qin Y , Lian Zhou X, Lai
Yang R, Hua Shi Y, Qing Mao H, et al. Diagnosis and treatment
of subclinical hypothyroidism detected by neonatal screening. World
J Pediatr. 2011;7:350-4.
25. Torun E, Cindemir E, Özgen IT, Öktem F.
Subclinical hypothyroidism in obese children. Dicle MedJ.
2013;40:5-8.
26. Cebeci AN, Güven A, Yýldýz M. Profile of
hypothyroidism in Down’s syndrome. J Clin Res Pediatr Endocrinol. 2013;5:116-20.
27. Soliman GZA, Bahagt NM, EL-mofty Z.
Prevalence of thyroid disorder in Egyptian children with type I
diabetes mellitus and the prevalence of thyroid antibodies among
them. Thyroid Disorders Ther. 2013;2:1.
28. Zulewski H, Müller B, Exer P, Miserez AR.
Estimation of tissue hypothyroidism by a new clinical score:
Evaluation of patients with various grades of hypothyroidism and
controls. J Clin Endocrinol Metab. 1997;82:771-6.
29. Gopalakrishnan S, Chugh PK, Chhillar M.
Goitrous autoimmune thyroiditis in a pediatric population: A
longitudinal study. Pediatrics. 2008;122:e670-4.
30. Radetti G, Gottardi E, Bona G, Corrias A,
Salardi S, Loche S, et al. The natural history of euthyroid
Hashimoto’s thyroiditis in children. J Pediatr. 2006;149:827-32.
31. Wasniewska M, Salerno M, Cassio A, Corrias A,
Aversa T, Zirilli G, et al. Prospective evaluation of the
natural course of idiopathic subclinical hypothyroidism in childhood
and adolescence. Eur J Endocrinol. 2009;160:417-21.
32. Radetti G, Maselli M, Buzi F, Corrias A,
Mussa A, Cambiaso P, et al. The natural history of the
normal/mild elevated TSH serum levels in children and adolescents
with Hashimoto’s thyroiditis and isolated hyperthyro-tropinaemia: A
3-year follow-up. Clin Endocrinol (Oxf). 2012;76:394-8.
33. Zois C, Stavrou I, Svarna E, Seferiadis K,
Tsatsoulis A. Natural course of autoimmune thyroiditis after
elimination of iodine deficiency in northwestern Greece. Thyroid.
2006;16:289-93.
34. Jaruratanasirikul S, Leethanaporn K,
Khuntigij P, Sriplung H. The clinical course of Hashimoto’s
thryoiditis in children and adolescents: 6 years longitudinal
follow-up. J Pediatr Endocrinol Metab. 2001;14:177-84.
35. Leonardi D, Polizzotti N, Carta A, Gelsomino
R, Sava L, Vigneri R, et al. Longitudinal study of thyroid
function in children with mild hyperthyrotropinemia at neonatal
screening for congenital hypothyroidism. J Clin Endocrinol Metab.
2008;93:2679-85.
36. Moore DC. Natural course of subclinical
hypothyroidism in childhood and adolescence. Arch Pediatr Adolesc
Med. 1996;150:293-7.
37. Wasniewska M, Corrias A, Aversa T, Valenzise
M, Mussa A, De Martino L, et al. Comparative evaluation of
therapy with L-Thyroxine versus no treatment in children with
idiopathic and mild subclinical hypothyroidism. Horm Res Paediatr.
2012;77:376-81.
38. Cetinkaya E, Aslan A, Vidinlisan S, Ocal G.
Height improvement by L-thyroxine treatment in subclinical
hypothyroidism. Pediatr Int. 2003;45:534-7.
39. Chase HP, Garg SK, Cockerham RS, Wilcox WD, Walravens PA. Thyroid
hormone replacement and growth of children with subclinical
hypothyroidism and diabetes. Diabet Med. 1990;7:299-303.

