|
|
|
Indian Pediatr 2012;49:
881-887 |
 |
Disease Course in Steroid Sensitive Nephrotic
Syndrome
|
|
Aditi Sinha, Pankaj Hari, Piyush Kumar Sharma, Ashima Gulati, Mani
Kalaivani*, Mukta Mantan, Amit Kumar Dinda †,
Rajendra N Srivastava and Arvind Bagga
From Departments of Pediatrics, *Biostatistics and
†Pathology, All India Institute of Medical
Sciences, New Delhi, India.
Correspondence and offprint requests to: Prof. Arvind
Bagga, MD, Department of Pediatrics, All India Institute
of Medical Sciences, Ansari Nagar, New Delhi 110029, India.
Email: [email protected]
Received: December 7, 2011;
Initial review: December 23, 2011;
Accepted: February 10, 2012.
Published
online: 2012, March 30.
PII:S097475591100999-1
|
Objective: To review the disease course in patients with steroid
sensitive nephrotic syndrome (SSNS) and the factors that determine
outcome
Design: Retrospective, analytical
Setting: Pediatric Nephrology Clinic at referral
center in North India
Participants/patients: All patients with SSNS
evaluated between 1990 and 2005
Intervention: None
Main outcome measures: Disease course, in
patients with at least 1-yr follow up, was categorized as none or
infrequent relapses (IFR), frequent relapses or steroid dependence (FR),
and late resistance. Details on complications and therapy with
alternative agents were recorded.
Results: Records of 2603 patients (74.8% boys)
were reviewed. The mean age at onset of illness and at evaluation was
49.7±34.6 and 67.5±37.9 months respectively. The disease course at 1-yr
(n=1071) was categorized as IFR in 37.4%, FR in 56.8% and late
resistance in 5.9%. During follow up, 224 patients had 249 episodes of
serious infections. Alternative medications for frequent relapses (n=501;
46.8%) were chiefly cyclophosphamide and levamisole. Compared to IFR,
patients with FR were younger (54.9±36.0 vs. 43.3±31.4 months),
fewer had received adequate ( ³8
weeks) initial treatment (86.8% vs. 81.7%) and had shorter
initial remission (7.5±8.6 vs. 3.1±4.8 months) (all P<0.001).
At follow up of 56.0±42.6 months, 77.3% patients were in remission or
had IFR, and 17.3% had FR.
Conclusions: A high proportion of patients with
SSNS show frequent relapses, risk factors for which were an early age at
onset, inadequate initial therapy and an early relapse.
Keywords: Frequent relapses; Minimal change disease; Steroid
dependent nephrotic syndrome.
|
|
The course of illness in
children with steroid sensitive nephrotic syndrome varies from a single
episode to infrequent or frequent relapses, and rarely the occurrence of
late steroid resistance [1,2]. The management of patients with frequent
relapses, steroid dependence and late resistance is difficult, often
requiring the use of alternative agents. Information on the course of
nephrotic syndrome is available from multiple cohorts, including that of
the International Study of Kidney Diseases in Children (ISKDC) [1,3-6].
Risk factors for frequent relapses include an early age of onset, short
initial therapy, delayed time to remission and brief duration of the
first remission [1,7-11]. An understanding of factors that determine the
course is useful in decisions regarding therapy and enables counseling.
In 1975, we described our experience on the clinical
features and renal histology in 206 consecutive patients with nephrotic
syndrome [12]. The findings suggested that clinical and histological
features of nephrotic syndrome in Indian children were similar to that
reported elsewhere [1]. While the management of the initial episode and
choice of alternative medications have changed over the years, there is
limited contemporary data on the outcomes. We reviewed the case notes of
recent patients, in order to examine the course of illness and its
determinants.
Methods
We retrospectively analyzed the records of all
patients with idiopathic steroid sensitive nephrotic syndrome, having an
Indian ancestry, who presented to this center between 1990 and 2005.
Children with onset of illness after 15-years, congenital nephrotic
syndrome (onset <3-months of age) or known to be secondary to infections
or systemic disease, or estimated glomerular filtration rate (eGFR)
£60 mL/min/1.73
m2 at evaluation were
excluded.
Disease course
The disease course, use of alternative therapies and
complications were described for patients with minimum 12-months follow
up at this center (Study Group). The course of disease during
these 12 months was categorized as single episode, infrequent relapses,
frequent relapses, steroid dependence or late resistance. Frequent
relapses was defined as the occurrence of ≥2 relapses in 6
months or
≥3
relapses in 12 months, and steroid dependence as the presence of two
consecutive relapses while on tapering doses of prednisolone [13].
Failure to show remission of proteinuria despite 4-weeks treatment with
prednisolone (2 mg/kg/day) was termed as initial resistance
when noted at onset of disease, and late resistance if occurring
in a patient previously responsive to steroids [13].
Hypertension was defined as blood pressure higher
than 95 th percentile for
sex, age and height [14]. Short stature was height less than 2 standard
deviations (SD) and obesity was body mass index more than 3 SD of
expected [15]. Patients had regular examination for visual acuity,
intraocular pressure and cataract. Standard definitions were used for
systemic infections [13]. Tuberculosis was diagnosed if the tuberculin
test was positive (induration ≥10 mm at 48 hr) in presence of
clinical and radiological features.
Therapy
Until 1992, patients at the first episode of
nephrotic syndrome were treated with daily prednisolone for 4 weeks
followed by alternate day therapy for 4 weeks. Between 1992 and 1995,
patients participating in a randomized controlled trial received the
aforementioned 8-weeks or prolonged 16-weeks treatment [16]. Thereafter,
patients have received therapy with daily and alternate day prednisolone
for 6 weeks each. For purpose of this review, adequate initial
treatment was the use of prednisolone (2 mg/kg/day) for
≥4 weeks followed by
1.5 mg/kg on alternate days for
≥4
weeks. Relapses were treated with prednisolone, 2 mg/kg/d until
remission and 1.5 mg/kg on alternate days for 4 weeks.
Patients with frequent relapses or steroid dependence
received prednisolone (0.3-0.7 mg/kg) on alternate days for 9-12 months.
Those having relapses or steroid toxicity received one or more
alternative agents [13], often as follows: (i) levamisole (2
mg/kg on alternate days); (ii) oral cyclophosphamide (2 mg/kg/d
for 12 weeks); (iii) mycophenolate mofetil (600-1000 mg/m 2/d);
(iv) cyclosporine (4-6 mg/kg/d) or tacrolimus (0.1-0.2 mg/kg/d).
Kidney biopsies were done in patients with persistent
hematuria, deranged renal function, steroid resistance or prior to
therapy with calcineurin inhibitors, and examined by light microscopy
and immunofluorescence [17].
Statistical analysis: Data from eligible patients
with steroid sensitive illness was used to compute baseline features.
Characteristics of patients in the Study Group were compared between the
infrequent relapsers (patients with single episode or infrequent
relapses) and frequent relapsers (frequent relapses or steroid
dependence). Analyses were performed using Stata 11 (Statacorp, College
Station, TX). Summary statistics were expressed as means and SD; groups
were compared using Student’s t test and Chi square test. On logistic
regression, risk factors were reported as odds ratio (OR) with 95%
confidence interval (CI). For this purpose, certain variables were
dichotomized based on information from previous studies [1,3,8,18,19].
These included the age at onset (<4 yr or
≥4 yr); duration of
initial therapy (<8 weeks or
≥8
weeks) and duration of remission following therapy of the initial
episode (<6 months or
≥6
months).
Results
Of 3299 patients with nephrotic syndrome, 324 were
excluded due to inadequate case records, age of onset >15 yr or presumed
secondary etiology (Fig. 1). Initial steroid resistance
was noted in 372 (12.5% of 2975) patients. Details on baseline
characteristics were available in 2603 patients with steroid sensitive
nephrotic syndrome (Table I). Most patients had received
adequate initial therapy with prednisolone and over one-half received
treatment for 12 weeks. The duration of initial remission was 4.6±6.4
months. Renal biopsies, in 341 patients, showed minimal change disease
in 242 (70.9%), mesangioproliferative glomerulonephritis in 48 (14.1%)
and focal segmental glomerulosclerosis in 51 (15.0%).
 |
|
Fig. 1 Flow chart showing patients
with nephrotic syndrome. The course of disease in patients with
minimum 12-months follow up at this center (Study Group) is
depicted.
|
TABLE I Baseline Features of Patients with Steroid Sensitive Nephrotic Syndrome
|
All patients;
|
Study Group;
|
|
N=2603 |
N=1071 |
|
Boys |
1947 (74.8%) |
816 (76.2%) |
|
Age at onset, mo
|
49.7 ± 34.6 |
48.7 ± 34.4 |
|
Age at evaluation, mo
|
67.5 ± 37.9 |
67.4 ± 39.2 |
|
Family history of disease |
46 (1.8) |
15 (1.4) |
|
Hematuria |
242 (9.3) |
85 (7.9) |
|
Hypertension |
97 (3.7) |
35 (3.3) |
|
Blood investigations
|
|
Creatinine, mg/dL
|
0.71 ± 0.34 |
0.70 ± 0.31 |
|
Albumin, g/dL
|
2.3 ± 0.9 |
2.2±0.9 |
|
Cholesterol, mg/dL |
343.2 ± 122.0 |
344.7 ± 122.6 |
|
Initial prednisolone therapy
|
|
Total duration, wks
|
13.6 ± 6.2 |
13.2 ± 6.2 |
|
Adequate therapy (³8 wks) |
2193 (84.3)
|
901 (84.1) |
|
8-11 wks
|
699 (26.9) |
318 (29.7) |
|
≥12 wks |
1494 (57.4) |
583 (54.4) |
|
Duration of initial remission (mo) |
4.6 ± 6.4 |
4.8 ± 7.0 |
|
Continuous variables are denoted as mean±standard deviation (95%
ci), categorical variables as n (%) |
Study Group
Of all patients with steroid sensitive nephrotic
syndrome, 1071 (41.2%) having minimum 12-months follow up at this center
constituted the Study Group. Following a mean age of onset of 48.7±34.4
months, and initial remission of 4.8±7.0 months, the age at evaluation
was 67.4±39.2 months (Table 1). At follow up of 12 months, the disease
course was categorized as none or infrequent relapses in 37.4%, frequent
relapses or steroid dependence in 56.8% and late resistance in 5.9% (Fig.
1).
Within the Study Group, 312 patients (237 boys;
76.0%) had been followed since onset of nephrotic syndrome. Their age at
onset was 46.7±33.9 months, and disease course during 12-months was
defined as none or infrequent relapses in 46.8%, frequent relapses in
49% and late resistance in 4.2%.
Alternative medications: These were administered
for frequent relapses in 501 (46.8%) patients. Two, three and four
medications were required in 219 (20.5%), 101 (9.4%) and 47 (4.4%)
instances respectively (Fig. 2). Most patients received
therapy with oral cyclophosphamide or levamisole initially, followed by
mycophenolate mofetil or a calcineurin inhibitor.
 |
|
Fig. 2 Alternative medications
for patients with frequent relapses or steroid dependence in the
Study Group. Heights of individual bars represent the
proportions of patients receiving the medication at each step of
therapy. The number (%) of patients is shown in the panel below.
|
Complications: A significant proportion of
patients in the Study Group showed adverse effects of corticosteroid
therapy. During a mean follow up of 56.0±42.6 months, 224 patients had
249 episodes of serious infections, which required hospitalization.
These included peritonitis (7.7%), pneumonia (5.3%), cellulitis
(3.7%), diarrheal dehydration (2.2%), urinary tract infections (2.1%)
and tuberculosis (1.8%). Sequelae of corticosteroid therapy included
obesity (n=226, 21.1%), hypertension (n=62, 5.8%),
stunting (n=53, 4.9%) and cataract (n=17, 1.6%). Behavior
abnormalities and thrombosis were observed in 38 (3.6%) and 4 (0.4%)
patients. Patients with frequent relapses had a significantly higher
risk of serious infections (OR 2.37; 95% CI 1.66, 3.39), obesity (OR
2.70; 1.92, 3.80), stunting (OR 2.64; 1.45, 4.79) and hypertension (OR
2.22; 1.14, 4.33).
TABLE II Characteristics of Infrequent Relapsers, Frequent Relapsers and Patients With Late Resistance
|
Infrequent relapsers† |
Frequent relapsers‡ |
Late resistance,
|
|
n=400 |
n=608 |
n=63
|
|
Boys |
306 (76.5)
|
474 (77.9)
|
44 (69.1) |
|
Age at onset, mo
|
54.9 ± 36.0 (51.6, 58.1) |
43.3 ± 31.4 (40.6, 46.0)** |
47.9 ± 39.3 (37.2, 58.5) |
|
Family history of disease |
6 (1.5) |
9 (1.5) |
0 (0) |
|
Hematuria at onset |
34 (8.5) |
41 (6.7) |
10 (15.9)#$ |
|
Investigations
|
|
Creatinine, mg/dL |
0.67 ± 0.23 (0.59, 0.72) |
0.64 ± 0.42 (0.57, 0.68) |
0.79 ± 0.77 (0.56, 1.03)
|
|
Albumin, g/dL
|
2.3 ± 0.9 (2.2, 2.4) |
2.2 ± 0.9 (2.2, 2.3) |
2.2 ± 0.8 (1.9, 2.4) |
|
Cholesterol, mg/dL |
347.3 ± 123.9 (331, 363) |
346.8 ± 121.7 (335, 359) |
328.4 ± 129.4 (286, 371) |
|
Initial steroid therapy
|
|
Total duration, wks |
13.7 ± 6.2 (13.1, 14.2) |
12.8 ± 6.1 (12.2, 13.3)* |
14.3 ± 7.4 (12.3, 16.4)
|
|
Adequate therapy (>8 wks) |
347 (86.8) |
490 (81.7)* |
53 (84.1) |
|
Duration of initial remission, mo |
7.5 ± 8.6 (6.5, 8.5) |
3.1 ± 4.8 (2.6, 3.5)** |
3.9 ± 7.7 (1.2, 6.5)## |
†Single episode or infrequent relapses; ‡Frequent relapses or
steroid dependence; Continuous variables are denoted as
mean±standard deviation (95% CI), categorical variables as n
(%); Difference between infrequent relapsers and frequent
relapsers: *P<0.05; **P<0.001; Difference between infrequent
relapsers and late resistance: #P<0.05,
##P <0.001; Difference between frequent relapsers and
patients with late resistance: $P<0.05. |
Risk factors for disease course: Table
II compares the characteristics of the frequent (n=608) and
infrequent relapsers (n=400). The former were younger at onset of
the disease (mean difference 11.5 months; 95% CI 7.3, 15.8 months; P<0.001)
and the proportion of patients with frequent relapses declined with
increasing age (Fig. 3). The duration of initial therapy
was short in frequent relapsers compared to those with infrequent
relapses (mean difference 0.9 weeks, 95% CI 0.1, 1.6 weeks; P=0.02).
A significantly lower proportion of frequent relapsers had received
adequate ( ³8
weeks) initial steroid therapy. Initial therapy for
³12 weeks was
associated with an additional 30% reduced risk of frequent relapses
compared to those treated for 8-11 weeks (OR 0.70; 95% CI 0.51, 0.95;
P=0.02). Frequent relapsers also had brief initial remission,
compared to those with infrequent relapses (mean difference 4.5 months;
95% CI 3.4, 5.5 months; P <0.001).
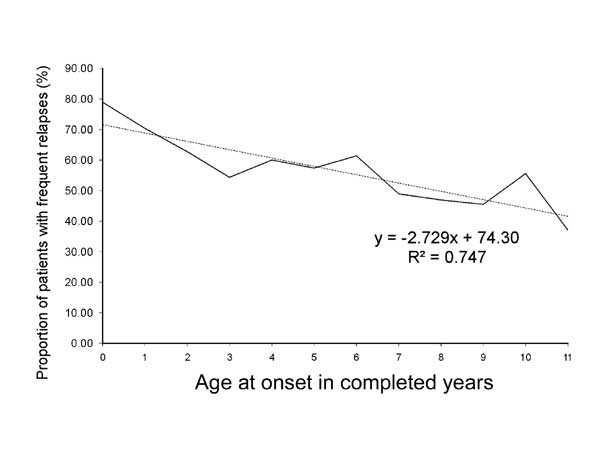 |
|
Fig. 3 Proportion of patients with
frequent relapses or steroid dependent nephrotic syndrome in
relation to the age at onset of the illness.
|
On univariate and multivariate logistic regression,
young age at onset (<4 yr), lack of adequate initial therapy (<8 weeks)
and short duration of initial remission (<6 months) were each associated
with significantly increased risk of frequent relapses (Table
III).
TABLE III Risk Factors for Frequent Relapses (N=1071)
|
Odds Ratio (95% CI) |
|
Unadjusted |
P |
Adjusted |
P |
|
Age at onset ≥4 y |
0.64 (0.49, 0.85) |
0.002 |
0.62 (0.43, 0.90) |
0.01 |
|
Initial therapy ≥8 weeks
|
0.61 (0.41, 0.91) |
0.015 |
0.55 (0.33, 0.92) |
0.02 |
|
Initial remission ≥6 months |
0.17 (0.11, 0.25) |
<0.001 |
0.18 (0.11, 0.27) |
<0.001 |
Late resistance: Of 1071 patients, 63 (5.9%)
showed late resistance. The presence of hematuria (gross 7; microscopic
3) (Table II) was independently associated with late
steroid resistance (OR 3.3, 95% CI 1.4, 8.1; P=0.007).
Compared to patients with infrequent relapses, the duration of initial
remission was shorter (mean difference 3.7 months, 95% CI 0.7, 6.7
months; P=0.02). Renal histology (n=54) showed
focal segmental glomerulosclerosis in 25 (46.3%), minimal change disease
in 22 (40.7%) and mesangioproliferative glomerulonephritis in 7 (13%).
Therapies included cyclosporine or tacrolimus in 40 (63.5%) patients and
IV cyclophosphamide in 23 (36.5%) patients; complete or partial
remission was seen in 39 (61.9%).
Outcome
Table IV shows the outcome of patients at
last follow up at 56.0±42.6 (range 12-160) months. Infrequent relapses
or sustained remission was seen in 828 (77.3%) patients, 185 (17.3%) had
frequent relapses or steroid dependence, and 42 (3.9%) showed late
steroid resistance. Most patients (89.8%) with infrequent relapses at
initial evaluation were in remission or had infrequent relapses. The
outcome in patients with frequent relapses or steroid dependence was
also satisfactory; 72.0% had remission or infrequent relapses and 23.8%
persisted with frequent relapses. At last follow up, 53.8% of 145
patients with frequent relapses continued to require alternative
immunosuppressive agents, most commonly levamisole (n=37) or
cyclophosphamide (n=23), while others (n=67, 46.2%) were
receiving low dose prednisolone on alternate days. A small proportion
(2%) having late steroid resistance was managed chiefly with calcineurin
inhibitors.
TABLE IV Outcome of Patients at Last Follow up (N=1071) in Relation to Initial Course.
|
Outcome at last follow up |
Infrequent relapses (n=400),
|
Frequent relapses (n=608),
|
Late resistance
|
|
No (%) |
No (%) |
(n=63) |
|
Sustained remission, infrequent relapses
|
359 (89.8) |
438 (72.0) |
31 (49.2) |
|
Frequent relapses, steroid dependence
|
32 (8.0) |
145 (23.8) |
8 (12.7) |
|
Late steroid resistance
|
9 (2.3) |
12 (2.0) |
21 (33.3) |
|
Died |
0
|
13 (2.1) |
3 (4.8) |
A significant proportion of patients (33.3%) with
late resistance had persistent nephrotic range proteinuria. In patients
with late resistance, the course of illness was similar in those with
focal segmental glomerulosclerosis, minimal change disease and
mesangioproliferative glomerulonephritis (data not shown). Sixteen
(1.5%) patients, with either frequent relapses or late resistance, died
of complications of severe infections. Eleven deaths occurred in
patients admitted with refractory septic shock following pneumonia (n=5),
diarrhea (n=3), meningitis with severe pneumonia (n=1) or
fever without focus (n=2). Five children died at home with
probable diagnosis of severe pneumonia.
Discussion
We report the course of illness in a large group of
patients with steroid sensitive nephrotic syndrome seen at a tertiary
care center in India during 1990 to 2005. Data from 1071 patients, with
minimum follow up of 12 months, was used to determine the course and
risk factors associated with frequent relapses. During this period,
practices regarding diagnosis and therapy were relatively constant,
except that MMF and tacrolimus were included as alternative agents after
1998. These findings are important since they reflect outcomes of
existing practice, as compared to most previous reports that include
data on patients diagnosed during 1970-80 [1,3-6,12].
The characteristics of our patients were similar to
that reported previously including age at onset, male preponderance and
low incidence of familial cases [1,12, 20]. The proportion of patients
having initial and late resistance was 12.5% and 5.9% respectively,
confirming previous findings [3,5,21]. While the ISKDC reported that
just 28.1% patients show frequent relapses in the first 6 months of
their illness [3], data from other centers, comprising relatively small
numbers of patients, shows that the proportion of frequent relapsers
varies from 56-68% [8,10-11]. The present case series suggests that,
despite 2-3 years from onset, frequent relapsers constitute more than
one-half of all patients. While this information might suggest a
referral bias, it is notable that 49% of the 312 patients followed at
this center since onset of disease had frequent relapses. The reasons
for detecting a high proportion of patients with frequent relapses are
unclear, but might reflect biologic variations in disease severity or
the increased occurrence of infection induced relapses [22].
There is evidence that adequate initial therapy with
corticosteroids is useful in reducing the risk of subsequent relapses
[23]. The present study also suggests that extension of initial therapy
to 8-11 weeks was associated with reduced proportion of patients with
frequent relapses; those receiving therapy for
≥12 weeks had an
additional 30% lower risk. While data from prospective studies, reviewed
recently [24], emphasize the benefits of prolonged therapy, its optimal
duration is not known and is being addressed in randomized controlled
trials.
Findings from the present study confirm that an early
age at onset of nephrotic syndrome is associated with risk of frequent
relapses [8,18,25]. Based on an age cut-off proposed in previous
studies, we found that patients with onset of illness beyond 4-yr had a
38% lower risk of frequent relapses as compared to younger children.
However, data from the ISKDC cohort [1] and others [10, 11] do not
report an association between age at onset and the occurrence of
frequent relapses. Finally, the risk of frequent relapses was reduced by
82% in children with initial remission lasting 6 months of longer,
supporting findings of the ISKDC report [1] and of Fujinaga, et al.
[19].
Almost one-half of the patients, who were followed
up, required alternative medications for frequent relapses. Conforming
to national guidelines and similar to practices elsewhere,
cyclophosphamide and levamisole were the preferred first-line therapies,
while the use of calcineurin inhibitors and mycophenolate mofetil was
limited [13,26]. The diminishing numbers of patients requiring
alternative medications at each step (Fig. 2) reflects the
changes in disease course with therapy and time. Our experience on the
impact of these treatment regimens has been reported earlier [27-29].
Long-term follow up of the ISKDC cohort, 9 years from
the onset, suggests that the tendency to relapse reduces with time, and
that most patients had either sustained remission or infrequent relapses
[3]. Five years after diagnosis, almost three-fourth patients in the
present study showed such a course, and only one-fifth continued to
either relapse frequently or had unremitting proteinuria. The outcomes
were similar in patients with frequent relapses and steroid dependence
(data not shown). Koskimies, et al similarly reported
sustained remission in 78.7% of 94 steroid sensitive patients at 5-14 yr
follow up [5]. In a cohort of 132 children, followed over 27 years,
Wynn, et al. found sustained remission in 62.8%; 17% patients had
died of renal causes [6].
The limitations of the present analysis are similar
to those of any retrospective report, including recall or reporting
bias. There was limited information on the time to first remission,
precise indications and duration of use of alternative agents, and on
morbidities that did not require hospitalization. Patients were referred
many months after the onset of symptoms and with a relatively difficult
illness, perhaps limiting the scope of these findings. However,
similarity of the disease course in patients who had presented at the
first episode suggests that these findings were valid and might be
generalized to patients with nephrotic syndrome in India.
The present study, on a large and recent group of
patients with steroid sensitive nephrotic syndrome, identifies the
course of the illness and existing therapeutic practices. It reconfirms
the importance of age at onset of nephrotic syndrome, the need for
adequate initial steroid therapy and duration of initial remission in
predicting the risk of frequent relapses. The outcomes were
satisfactory, and on follow up most patients were in sustained remission
or had infrequent relapses.
Contributors: RNS, AB and PH were responsible for
setting up the database. AS, PKS, AG, MM were involved in retrieval of
information from records and its analysis, MK provided statistical
inputs, and AKD reported all histological specimens. All authors
contributed to the preparation of the manuscript, provided significant
inputs during preparation for final publication and approved the final
manuscript. AB supervised the study and shall be its guarantor.
Funding: None; Competing interests: None
stated.
|
What Is Already Known?
• Frequent relapses are an important cause of
morbidity in children with steroid sensitive nephrotic syndrome.
• Risk factors for relapses are an early age
of onset, short initial therapy and delayed time to remission.
What This Study Adds?
• More than one-half of all patients with
steroid sensitive nephrotic syndrome show frequent relapses.
Risk factors include onset at <4 years of age, initial therapy
for less than 8 weeks, and brief initial remission lasting <6
months.
• Infections constitute the chief cause for
morbidity and mortality.
• The long-term outcome of nephrotic syndrome is satisfactory
in the majority.
|
References
1. Report of the International Study of Kidney
Disease in Children. Early identification of frequent relapsers among
children with minimal change nephrotic syndrome. A report of the
International Study of Kidney Disease in Children. J
Pediatr.1982;101:514-8
2. Bagga A, Mantan M. Nephrotic syndrome in children.
Indian J Med Res. 2005;122:13-28.
3. Tarshish P, Tobin JN, Bernstein J, Edelman CM.
Prognostic significance of the early course of minimal change nephrotic
syndrome: Report of the International Study of Kidney Disease in
Children. J Am Soc Nephrol. 1997;8:769-76.
4. Fakhouri F, Bocquet N, Taupin P, Presne C,
Gagnadoux MF, Landais P, et al. Steroid-sensitive nephrotic
syndrome: From childhood to adulthood. Am J Kidney Dis. 2003; 41:550-7.
5. Koskimies O, Vilska J, Rapola J, Hallman N.
Long-term outcome of primary nephrotic syndrome. Arch Dis Child.
1982;57:544-8.
6. Wynn SR, Stickler GB, Burke EC. Long-term
prognosis for children with nephrotic syndrome. Clin Pediatr.
1988;27:63-8.
7. Hodson EM, Willis NS, Craig JC. Non-corticosteroid
treatment for nephrotic syndrome in children. Cochrane Database Syst
Rev. 2008;1: CD002290; DOI 10.1002/14651858.CD002290.pub3
8. Andersen RF, Thrane N, Noergaard K, Rytter L,
Jespersen B, Rittig S. Early age at debut is a predictor of
steroid-dependent and frequent relapsing nephrotic syndrome. Pediatr
Nephrol. 2010;25:1299-1304.
9. Letavernier B, Letavernier E, Leroy S, Baudet-Bonneville
V, Bensman A, Ulinski T. Prediction of high-degree steroid dependency in
pediatric idiopathic nephrotic syndrome. Pediatr Nephrol.
2008;23:2221-6.
10. Yap HK, Han EJS, Heng CK, Gong WK. Risk factors
for steroid dependency in children with idiopathic nephrotic syndrome.
Pediatr Nephrol. 2001;16:1049-52.
11. Constantinescu AR, Shah HB, Foote EF, Weiss LS.
Predicting first-year relapsers in children with nephrotic syndrome.
Pediatrics. 2000;105:492-5.
12. Srivastava RN, Mayekar G, Anand R, Choudhry VP,
Ghai OP, Tandon HD. Nephrotic syndrome in Indian children. Arch Dis
Child. 1975;50:626-30.
13. Indian Pediatric Nephrology Group, Indian Academy
of Pediatrics. Management of steroid sensitive nephrotic syndrome:
Revised guidelines. Indian Pediatr. 2008;45:203-14.
14. National High Blood Pressure Education Program
Working Group on High Blood Pressure in Children and Adolescents. The
fourth report on the diagnosis, evaluation, and treatment of high blood
pressure in children and adolescents. Pediatrics. 2004;114:555-76.
15. Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche
AF. NCHS growth curves for children birth -18 years. United States.
Vital Health Stat 11. 1977;165:1-74.
16. Bagga A, Hari P, Srivastava RN. Prolonged versus
standard prednisolone therapy for initial episode of nephrotic syndrome.
Pediatr Nephrol. 1999;13:824-7.
17. International Study of Kidney Disease in
Children. Primary nephrotic syndrome in children: clinical significance
of histopathologic variants of minimal change and of diffuse mesangial
hypercellularity. Kidney Int. 1981;20:765-71.
18. Kabuki N, Okugawa T, Hayakawa H, Tomizawa S,
Kasahara T, Uchiyama M. Influence of age at onset on the outcome of
steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 1998;12:467-70.
19. Fujinaga S, Hirano D, Nishizaki N. Early
identification of steroid dependency in Japanese children with
steroid-sensitive nephrotic syndrome undergoing short-term initial
steroid therapy. Pediatr Nephrol. 2011;26:485-6.
20. Bakkali LE, Pereira RR, Kuik DJ, Ket JCF, van
Wijk JAE. Nephrotic syndrome in The Netherlands: a population-based
cohort study and a review of the literature. Pediatr Nephrol.
2011;26:1241-6.
21. Srivastava RN, Agarwal RK, Moudgil A, Bhuyan UN.
Late resistance to corticosteroids in nephrotic syndrome. J Pediatr.
1986;108:66-70.
22. Gulati A, Sinha A, Sreeniwas V, Math A, Hari P,
Bagga A. Daily corticosteroids reduce infection associated relapses in
frequently relapsing nephrotic syndrome: a randomized controlled trial.
Clin J Am Soc Nephrol. 2011;6:63-9.
23. Ehrich JH, Brodehl J. Long versus standard
prednisone therapy for initial treatment of idiopathic nephrotic
syndrome in children. Arbeitsgemeinschaft fur Pädiatrische Nephrologie.
Eur J Pediatr. 1993;152:357-61.
24. Hodson EM, Knight JF, Willis NS, Craig JC.
Corticosteroid therapy for nephrotic syndrome in children. Cochrane
Database Syst Rev. 2005;1:CD001533
25. Takeda A, Matsutani H, Niimura F, Ohgushi H. Risk
factors for relapse in childhood nephrotic syndrome. Pediatr Nephrol.
1996;10:740-1.
26. Abeyagunawardena AS, Dillon MJ, Rees L, van’t
Hoff W, Trompeter RS. The use of steroid-sparing agents in
steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2003;18:919-24.
27. Srivastava RN, Agarwal RK, Choudhry VP, Moudgil
A, Bhuyan UN, Sunderam KR. Cyclophosphamide therapy in frequently
relapsing nephrotic syndrome with and without steroid dependence. Int J
Pediatr Nephrol. 1985;6:245-50.
28. Bagga A, Sharma A, Srivastava RN. Levamisole
therapy in corticosteroid dependent nephrotic syndrome. Pediatr Nephrol.
1997;11:415-7.
29. Bagga A, Hari P, Moudgil A, Jordan SC.
Mycophenolate mofetil and prednisolone therapy in children with
steroid-dependent nephrotic syndrome. Am J Kid Dis. 2003;42:1114-20.
|
|
|
 |
|

